Darrent Antono
Contents
Chat With ChatGPT
In summary, the correlation between a sugar cane company, battery plant, and fuel is related to the use of sugar cane and its byproducts, such as bagasse, to produce ethanol. Sugar cane can be used to produce ethanol, which can be used as a fuel or as a component of battery electrolytes in some types of batteries. Additionally, bagasse can be used as a fuel in sugar cane processing to generate electricity, highlighting the interconnectedness of different industries in the production and use of various types of energy and materials
ECS 02 Hands on Project Synopsis: Virtual Analysis of I.C.E. with hybrid assist or hydrogen alternative fuel
In the modern age, the advent of hybrid or electric powered vehicles are clearer as ever. With the rise of global warming and the real danger of increasing seawater levels, stringent emission standards are becoming the norm. Manufacturers have battled for the sure-fire solution to this slow outgoing crisis, one of them being the use of hybrid powertrains and electric; half-battery, half combustion engine, or full-battery, zero combustion engine. Today, the use of electric batteries have proven to be quite popular. However, many have stated the looming danger of battery use. Studies suggest that electric battery powerplants produce 74% more carbon pollution than a collective bunch of gasoline-engined vehicles. The problem is made more dire by the possible waste clutter of lithium-ion when they've reached the end of the cycle, further causing major environmental concerns. Hence, it can be said that internal combustion engines will still live for another day, another century perhaps even. Hybrid technology utilizes motors that could help with the total energy use of internal combustion engines, by shifting light and heavy work between their electric motors and its integrated internal combustion engine.
Another new discovery in the world of automotive engineering is the use of water-based hydrogen fuels to power up internal combustion engines. Toyota Motor Company are constantly improving this technology by utilizing it on their endurance racing cars and quite recently on a "retro revival" project of their popular 80s sports compact car, the Sprinter Trueno. By using hydrogen instead of fossil fuel-based gasoline, the combustion process happens entirely with water, resulting in zero pollutants and almost no combustion waste products as we've seen with gasoline vehicles. This hands on project strives to study the inner workings and components of both hybrid and hydrogen engine cars by video analysis. By the end of the study, I hope that I'm able to further understand the workings of these two powerplants and how they could be beneficial in the long run of automotive engines.
Today's Notes: 28 Feb 2023
- What is the difference between pyrolysis and I.C.E.? - internal combustion engines use liquid fuel, meanwhile pyrolysis converts energy from solid products - Fuel occurs when there's a reaction of a certain substance to the environment, specifically carbon - Energy conversion system and environment issues focus on vibrations - Fuel can be made out of plastic
Today's Summary: 7 Maret 2023
There are 4 main topics that are taught today: Desalination, biomass, pyrolysis, and driers. Desalination is a process that reduces seawater into salt. Biomass are essentially products that are made out of biomaterial, and can be used to power an energy source or become the source of energy for a certain object or tool. pyrolysis is a process which decomposes materials using heat and high temperatures. In this subject, we also learn about the use of graphene as a means to filtrate particles, supposedly water. Graphene itself is a single-layer of carbon atoms tightly wounded to a hexagonal shape. There have been many studies regarding graphene, one of them including its use on electrical appliances due to its stong nature, which is apparently stronger than steel, and its high flexibility. In industries, renewable energies are becoming the norm, and gasification proves to be a source which could supply renewable energy to source gas in gas industries, gasification itself is a form of pyrolysis as well. Desalination alone also has a few number of issues, since the waters have been mostly contaminated with trash, many companies have tried to rectify this problem by deploying trash boats. In dire situations, we can use desalination to obtain water sources, such as countries or cities that have barren lands. Filtration can be used to enhance desalination, by means of reverse osmosis.
Project Update
For this update, we have made a small program to figure out the amount of hydrogen gas produced, the program is as follows:
- Electrolysis of water to capture hydrogen gas
- Constants
FARADAY_CONSTANT = 96485 # coulombs/mol MOLAR_MASS_H2 = 2.016 # g/mol NUMBER_OF_ELECTRONS = 2 # number of electrons transferred in the reaction
- Inputs
applied_voltage = float(input("Enter the applied voltage in volts: ")) current = float(input("Enter the current in amperes: ")) time = float(input("Enter the time in seconds: "))
- Calculation of hydrogen gas produced
charge = current * time # coulombs moles_of_electrons = charge / (FARADAY_CONSTANT * NUMBER_OF_ELECTRONS) moles_of_H2 = moles_of_electrons mass_of_H2 = moles_of_H2 * MOLAR_MASS_H2
- Output
print(f"Mass of hydrogen gas captured: {mass_of_H2:.3f} g")
Quiz 2
Below is the result of my second quiz:

Personal Project Report: Electrolysis and ICE
What is electrolysis?
Electrolysis is an experiment done to observe the changes in electron behavior due to current change. Essentially, electrolysis is used to separate or decompose the electrons of a compound molecule. In this experiment, we use electrolysis to separate water and sodium bicarbonate, in hopes that we can obtain hydrogen gas out of electrolysis and try to analyze how it is implemented in an ICE engine.
Materials and Apparatus
For this project, what we'll need is: Materials: 1. Aluminum plates 2. Container box (15 L) 3. Drafts 4. Used water bottles 5. Garden hoses 6. Sodium bicarbonate 7. Balloons
Apparatus: 1. Drill 2. Cutters 3. Beaker glass 4. Power supply (12V/5A and 12V/2A) 5. Cathode and anode connectors
Procedures 1. Drill holes on the box container to accommodate pipes, drafts, and a cathode cover. 2. Glue pipes, drafts, and cathode cover into the container 3. Insert cathode steels and anode steels into the drafts 4. Pour the container with water, and mix with sodium bicarbonate 5. Using a 12V/5A power supply, connect the power lines into cathode and anode poles 6. Insert a balloon into the cathode pipe. This will store the hydrogen 7. Turn on the power, and wait 30 minutes for hydrogen to form
Results From the results we can see that the more amperes are put into the system, the more power is produced.
Here is the link to our project PowerPoint presentation: [1]
Final Project Report
The experiment was based on a simple electrolysis experiment. We created a hydrogen generator that is capable of producing hydrogen particles from the electrolyte of sodium bicarbonate. The metal plates made from aluminum/stainless steel are used as conductors connecting the anode to the cathode of the power source and then the byproduct of Hydrogen at the Cathode and Oxygen at the Anode. The Hydrogen gas is then stored in a mineral water bottle. The objectives of this experiment was to Learn how hydrogen production can be established using simple tools, learn and understand the process of hydrogen production, learn how hydrogen can be stored in systems, and to obtain hydrogen gasses through the use of hydrolysis.
The setup consists of a plastic container that is drilled on the sides to fit the rod and then glued to make it leak proof. The top lid of the container is also drilled to make holes for the hose’s output hole. The hose is then connected to a bottle where then the generated hydrogen gas will gather. The making of the electrolysis kit went relatively well. However, the process of gluing the hoses and the drafts turns out to be quite the challenge. We have to make sure that the container box, which has been drilled beforehand, stays watertight despite the new holes. Hence, a lot of glue had been used to cover up the gaps formed. A lot of glue was also used to form a watertight seal on the hydrogen gas cover, which is made by cutting the top portion of a mineral water gallon and placing it on top of one of the metal plates. The reason behind excessive glue usage on this part was to ensure that the maximum amount of hydrogen can flow into the mineral water bottle with as little hydrogen escaping as possible. Separation of the anode/cathode plates were done using screw nuts, where they provide sufficient space between the metal plates so as to not disturb the electrolysis process. In model experiments, it is preferred to use NaCl (sodium chloride), which is salt. However, upon further research, we found out that the by-product of salt consists of chlorine gas, which is undoubtedly a hazardous substance to breathe. Hence, we used sodium bicarbonate to create the electrolyte, as it produces carbon dioxide as a by-product which does not harm the user during the experiment.
When the kit was activated and the anodes and cathodes were connected in the right places, it was observed that the cathode part of the metal plates produced bubbles, hence indicating hydrogen production. An extra nozzle and one way valve was integrated into the mineral water bottle hydrogen storage compartment where its purpose was to ensure safe ignition testing of the hydrogen. Despite the proof of hydrogen production, the amount that was created was so little that it wasn’t enough to ignite the nozzle.
Due to some factors that may play a role in the result, therefore there may be some complications when gathering these data. This includes the power source not meeting the capability of the maximum production of hydrogen gas bubbles, although it is observed to produce gaseous hydrogen bubbles, they seem to not match the pressure needed for the nozzle or the one-way switch valve. There may also be leakage throughout the whole hydrogen generator system, whether it be from the top of the system or the leak at the attachment holes of the rubber tubings. Overall, the hydrogen gas bubbles do not meet the max production rate, therefore the pressure isn’t enough to go through the one-way valve and the nozzle to create a hydrogen torch as expected.
In conclusion, Hydrogen production is possible with this electrolysis kit, it will take a long time for a large amount of hydrogen to be formed and with a small amount of voltage, very little hydrogen can be produced.
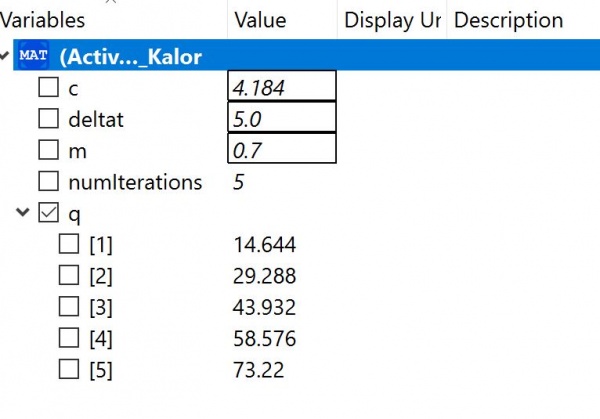
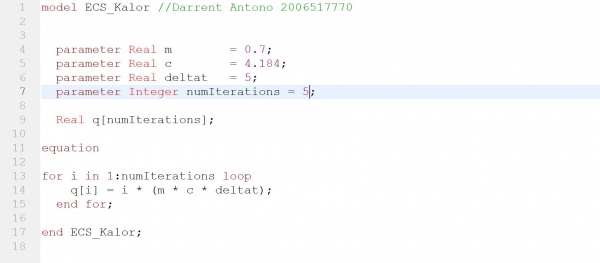
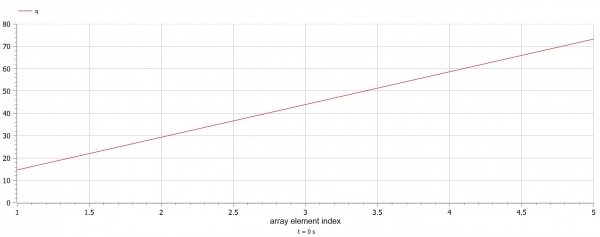
OpenModelica Program
In this program, we have three parameters, M, C, and delta T. M referring to the mass of the plates, C referring to the specific heat capacity of the water, and delta T referring to the temperature difference. Using this formula and an iteration method we can find out the amount of joules generated. The results show that 73.22 joules are generated through a temperature difference of 5 degrees.