Difference between revisions of "Julian Jensen Purnomo"
(→Introduction) |
(→Introduction) |
||
| Line 5: | Line 5: | ||
My class is at '''Numerical Method 03''' and my NPM is '''2106732834''' | My class is at '''Numerical Method 03''' and my NPM is '''2106732834''' | ||
| − | [[File: | + | [[File:JulianJensenPurnomo.jpg]] |
| + | |||
| + | I am currently on a journey to study Numerical Method with concious learning, so that I can improve my conciousness and become a better human. | ||
== Design and Optimization of 1 Litre Pressurized Hydrogen (8 Bar) Storage == | == Design and Optimization of 1 Litre Pressurized Hydrogen (8 Bar) Storage == | ||
Revision as of 01:23, 29 May 2023
Introduction
Hello everyone! My name is Julian Jensen Purnomo, usually called Julian. My class is at Numerical Method 03 and my NPM is 2106732834
I am currently on a journey to study Numerical Method with concious learning, so that I can improve my conciousness and become a better human.
Design and Optimization of 1 Litre Pressurized Hydrogen (8 Bar) Storage
-What is Hydrogen? Hydrogen is a chemical element with the symbol H and atomic number 1. It is the lightest and most abundant element in the universe, making up about 75% of its elemental mass. Hydrogen is a colorless, odorless, and tasteless gas in its pure form.
Hydrogen is unique because it has only one proton and one electron, making it the simplest and smallest atom. It is located in the first group of the periodic table, known as the alkali metals, but it is often placed separately due to its unique properties.
In nature, hydrogen is commonly found in combination with other elements, such as oxygen in water (H2O) or carbon in hydrocarbons. It is also present in the atmosphere, although in relatively small amounts. Hydrogen can be extracted from these sources through various methods, such as electrolysis of water or steam methane reforming.
Hydrogen has a wide range of applications. It is used as a fuel for rockets and in the production of ammonia for fertilizers. It can be used as a clean energy carrier and fuel source for fuel cells, which convert hydrogen into electricity through a chemical reaction with oxygen. Hydrogen fuel cells are being explored as a potential alternative to fossil fuels in vehicles and power generation.
This is LMP (Lemans Prototype) Car is powered by Hydrogen.
-How can we Store Hydrogen? Hydrogen can be stored in different forms, depending on the intended application and storage duration. Here are some common methods of hydrogen storage:
Compressed Gas: Hydrogen gas can be stored under high pressure in specially designed tanks or cylinders. The gas is compressed to pressures ranging from 5,000 to 10,000 psi (pounds per square inch). Compressed gas storage is commonly used for short-term applications and in situations where a relatively small amount of hydrogen is needed.
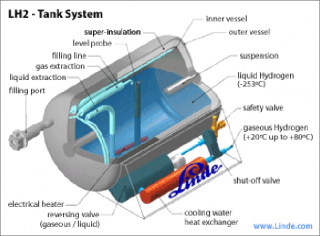
Liquid Hydrogen: At extremely low temperatures below -252.87°C (-423.17°F), hydrogen can be converted into a liquid state. Liquid hydrogen has a higher energy density compared to compressed gas, allowing for more hydrogen to be stored in a given volume. It is stored in cryogenic tanks that are well-insulated to maintain the low temperatures required.
This is a liquid Hydrogen Storage Tank.
Metal Hydrides: Hydrogen can be stored in certain metals or alloys, forming compounds known as metal hydrides. These materials have the ability to absorb and release hydrogen. Metal hydrides offer a safe and compact storage option, particularly for stationary and portable applications. However, they may have limited storage capacity and can be relatively heavy.
Chemical Hydrides: Chemical hydrides are compounds that release hydrogen when they undergo a chemical reaction. They can store a high amount of hydrogen per unit weight, but the release and reabsorption of hydrogen often require specific conditions, such as heating or the use of catalysts.
Carbon-Based Materials: Hydrogen can be stored within certain carbon-based materials, such as activated carbon or carbon nanotubes. These materials have a high surface area and can adsorb hydrogen molecules, effectively storing them within their structure. Research is ongoing to develop efficient and cost-effective carbon-based storage systems.
Each storage method has its advantages and limitations, and the choice depends on factors like storage capacity, safety, cost, and application requirements. Developing efficient and practical hydrogen storage systems is an important area of research to facilitate the widespread adoption of hydrogen as an energy carrier.