Difference between revisions of "Muhammad riffat muntaz heidir"
Riffatmuntaz (talk | contribs) |
Riffatmuntaz (talk | contribs) |
||
| Line 68: | Line 68: | ||
The result creates Aluminium Hydroxide and, of course, Hydrogen, which is the substance that we need to power up the Internal Combustion Engine. | The result creates Aluminium Hydroxide and, of course, Hydrogen, which is the substance that we need to power up the Internal Combustion Engine. | ||
| − | [[File:plat1.jpeg|250px]] | + | [[File:plat1.jpeg|250px|thumb|center|5 x 15cm Aluminium 1100 6mm]] |
| − | + | [[File:Alatrasen.jpeg|250px|thumb|center|Rasen's Hydrolysis Device]] | |
| − | |||
| − | + | I use 3 5x15 cm 6mm Aluminium 1100 plates for this experiment, and another parameter is the Amps which are 10 A, 8 A, and 6A. | |
| + | |||
| + | Below are the results for each different parameter: | ||
| + | |||
| + | |||
| + | [[File:H2.jpeg|400px|thumb|center|10 amps = 120 ml/min]] | ||
| + | |||
| + | |||
| + | [[File:H3.jpeg|400px|thumb|center|8 Amps = 90 ml/min]] | ||
| − | + | [[File:H1.jpeg|400px|thumb|center|6 Amps = 75 ml/min]] | |
| + | |||
| + | After obtaining the flow rate from the experiment, we can now build a model in Open Modelica using the data. We can compare the power generated from each different parameter. | ||
| + | |||
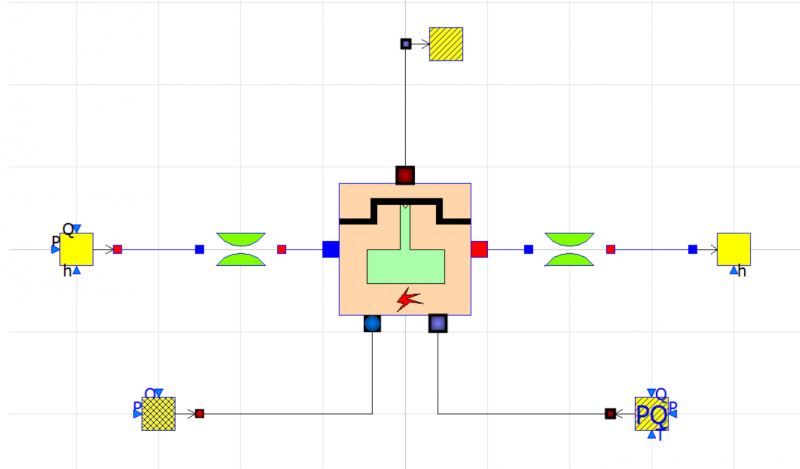
| + | [[File:OMEOME.png|400px|thumb|center|IC Engine Model]] | ||
| + | |||
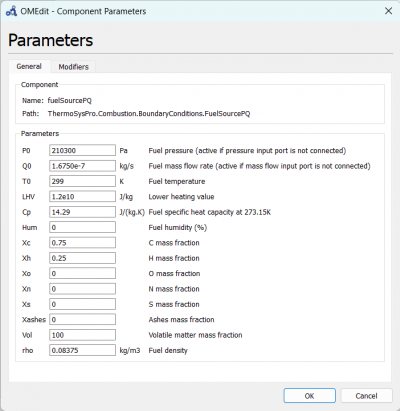
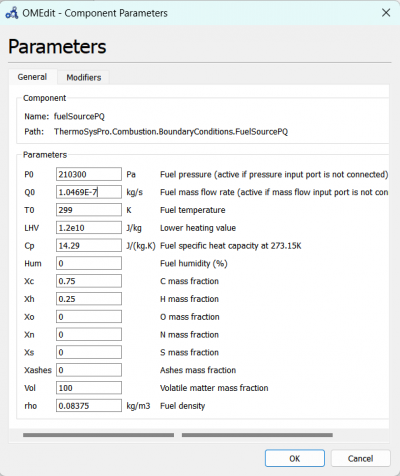
| + | We can change the parameters for fuelSourcePQ to the desired fuel source, which is Hydrogen and change the flow rate according to the experiment data. | ||
| + | [[File:OMEPAR.png|400px|thumb|center|fuelSourcePQ Parameter for 10 A]] | ||
| − | [[File: | + | [[File:OMEPAR2.png|400px|thumb|center|fuelSourcePQ Parameter for 8 A]] |
| − | + | [[File:OMEPAR3.png|400px|thumb|center|fuelSourcePQ Parameter for 6 A]] | |
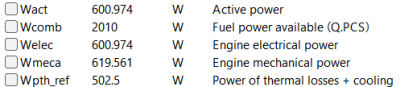
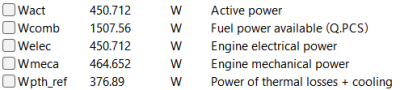
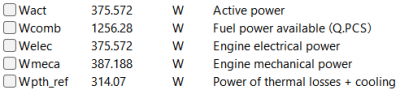
| − | + | After running the simulation, the result obtained is: | |
| − | + | [[File:OMERES.png|400px|thumb|center|Result for 10 A]] | |
| − | [[File: | + | [[File:OMERES2.png|400px|thumb|center|Result for 8 A]] |
| − | 6 | + | [[File:OMERES3.png|400px|thumb|center|Result for 6 A]] |
Latest revision as of 12:08, 11 April 2023
Contents
Introduction
Name : Muhammad Riffat Muntaz Heidir
NPM : 2006517814
e-mail : muhammad.riffat@ui.ac.id
Chat With ChatGPT
riffatmuntaz: what is the relation between sugarcane industry, car battery industry and petroleum industry
ChatGPT: There is a indirect relationship between the sugarcane industry, car battery industry, and petroleum industry.
The sugarcane industry is a source of renewable energy as it can be used to produce ethanol, which is a biofuel that can be blended with gasoline and used as a fuel for vehicles. The production of ethanol from sugarcane can help reduce the demand for petroleum-based fuels, which can have a positive impact on the petroleum industry.
The car battery industry, on the other hand, is not directly related to the sugarcane industry, but it does have a connection to the petroleum industry. This is because many car batteries are still made using lead-acid, which requires lead as a primary component. The production of lead involves the use of petroleum-based products, such as gasoline and diesel, which can have an impact on the petroleum industry.
In summary, while the sugarcane industry has the potential to reduce the demand for petroleum-based fuels, and the car battery industry has a connection to the petroleum industry, the relationships between these industries are not direct.
Modelling Internal Combustion Engine Using Open Modelica
Components:
- 1. alternatingEngine
- 2. fuelSourcePQ
- 3. sourceAir
- 4. sink
- 5. SourcePQ_Water
- 6. Sink_Water
- 7. singularPressureLoss
Internal Combustion Engine Personal Project: Motor Generator Unit - Heat (MGU-H)
MGU-H (Motor Generator Unit - Heat) is a component of the power unit in a Formula One (F1) car. It is part of the Energy Recovery System (ERS), which recovers energy that would otherwise be lost during braking and through exhaust gases.
The MGU-H converts heat energy from the exhaust gases into electrical energy, which can be used to power the car. It also acts as a generator, using electrical energy to drive a shaft which can spin the turbocharger.
In F1, the MGU-H is limited regarding how much energy it can recover and deploy during a race. The regulations dictate that drivers are only allowed to use 120 kilowatts (kW) of electrical power during a race, and the MGU-H is a crucial component in achieving this.
The MGU-H is an essential aspect of F1 racing, as it contributes to the overall efficiency and performance of the car. By recovering energy that would otherwise be lost, teams can gain a competitive advantage on the track. Additionally, developing the MGU-H and other ERS components is an area where F1 teams can showcase their technical expertise and innovation.
Class Summary 07/03/2023
Desalination is a method for removing minerals from saltwater (water containing a high concentration of dissolved salt). Desalination more broadly refers to the process of eliminating minerals and salts from a target substance. Desalination is the process used to provide drinking water for people from saltwater, particularly seawater. It must be filtered in order to be suitable for human consumption; one of the most recent discoveries is the use of a graphene filter.
Not only is graphene useful for desalination, but it can also be used for carbon capture to solve a different environmental problem (CCU). CCU's objective is to gather carbon dioxide (CO2) emissions from various sources and convert them into useful products like minerals, chemicals, or fuels. Biomass is a material derived from recently living creatures that is used to create bioenergy.
Biomass is made up of resources derived from living creatures like plants and animals because it is organic. The three types of biomass resources that are most frequently used as energy sources are plants, wood, and trash. They are what we refer to as biomass feedstocks. Biomass energy can also be a non-renewable energy source. Biochar is a type of biomass, but what is it exactly? Organic waste from forestry and agricultural operations is burned in a controlled process called pyrolysis to create biochar, a substance that resembles charcoal. Although it looks similar to conventional charcoal, biochar is made in a specific way to minimize contamination and effectively capture carbon.
Project Progress
Me and my friends bought a large sheet of Aluminium 1100 with 0.6mm thickness as it is considerably cheap, especially as we divide it for several people. We then cut the sheet into different sizes for the project. This is the video of us cutting the Aluminium 1100 sheet.
https://youtube.com/shorts/sqpt_SiQXSA?feature=share
Personal Project: Midterm Report
Hydrolysis is a chemical reaction that involves the use of water to break down a compound into simpler substances. A material reacts with water to create two or more products in this reaction. The process of hydrolysis involves the splitting of a water molecule into two hydrogen ions (H+) and a hydroxide ion (OH-), which subsequently react with the substance to break it down.
Salts, acids, and esters are just a few of the many types of chemicals that can undergo hydrolysis. For instance, sodium chloride (NaCl), a salt, hydrolyzes when it is dissolved in water to create sodium ions (Na+) and chloride ions (Cl-). Similar to this, when an acid dissolves in water, like hydrochloric acid (HCl), it goes through hydrolysis and creates hydrogen ions (H+) and chloride ions (Cl-).
For this personal project, I use Aluminium as the anode. As I used Aluminium 1100 for this experiment, it can be said that the Aluminium I used is relatively pure. Therefore, when Aluminium reacts with water, the reaction will be:
2Al + 6H2O = 2Al(OH)3 + 3H2
The result creates Aluminium Hydroxide and, of course, Hydrogen, which is the substance that we need to power up the Internal Combustion Engine.
I use 3 5x15 cm 6mm Aluminium 1100 plates for this experiment, and another parameter is the Amps which are 10 A, 8 A, and 6A.
Below are the results for each different parameter:
After obtaining the flow rate from the experiment, we can now build a model in Open Modelica using the data. We can compare the power generated from each different parameter.
We can change the parameters for fuelSourcePQ to the desired fuel source, which is Hydrogen and change the flow rate according to the experiment data.
After running the simulation, the result obtained is: