Difference between revisions of "Ignatius Adrian"
Adrian4649 (talk | contribs) (→Personal Project Report: Electrolysis and ICE) |
|||
| (9 intermediate revisions by one other user not shown) | |||
| Line 22: | Line 22: | ||
The automotive factories need the natural gas and gasoline of the oil and gas companies to create vehicles. | The automotive factories need the natural gas and gasoline of the oil and gas companies to create vehicles. | ||
| − | + | == Friday, 24 February 2023 == | |
Today, we learned how to create an alternating engine (internal combustion engine) using OpenModelica. Before creating the model, we had to install the ThermosysPro library, only after that will we be able to create the model. After installing the library, we opened a new model file and proceeded to import the Alternating Engine model from the library we just downloaded. | Today, we learned how to create an alternating engine (internal combustion engine) using OpenModelica. Before creating the model, we had to install the ThermosysPro library, only after that will we be able to create the model. After installing the library, we opened a new model file and proceeded to import the Alternating Engine model from the library we just downloaded. | ||
| Line 32: | Line 32: | ||
Using the reference from CCIT, we can then use the variables off of the reference. | Using the reference from CCIT, we can then use the variables off of the reference. | ||
| + | |||
| + | |||
| + | == Project Proposal == | ||
| + | For my personal project, I propose that we switch to electric-powered cargo ships. This idea falls into the EV Category. Cargo ships contributes 3.5% to 4% of all climate change emissions, mostly carbon dioxide. The use of electric-powered cargo ships should be able to reduce global warming. | ||
| + | |||
| + | The World Bank stated that, cargo ships are the 6th biggest contributor towards the world's carbon emission. This is because cargo ships operate near 24/7, produces a lot of carbon dioxide because of the fuel consumed in transport, and the sheer amount of existing cargo ships. The use of electric power as a substitute will bring great benefits to our planet. An electric cargo ship can be powered by a 6.8MWH battery, which is approximately a hundred times the size of an electric car's battery. The power is mainly harvested off hydro power. Using an average cargo ship's dimensions and weight, I can then model a cargo ship using a battery on OpenModelica and compare the emissions generated by a fully electric cargo ship. <br> | ||
| + | |||
| + | |||
| + | == Tuesday, 28 February 2023 == | ||
| + | |||
| + | |||
| + | Today, Professor Adi explained to us regarding ICE, Pyrolysis, and EV. | ||
| + | We talked about how combustion occurs, how it requires oxygen and carbon, and as a byproduct CO2 and water is produced. | ||
| + | Pyrolysis is the process in which waste is burned and converted into biofuel. The waste which can be converted into biofuel are organic materials such as biomass. | ||
| + | Combustion and pyrolysis is based off the process that is the ignition of carbon. | ||
| + | |||
| + | Mr DAI told us that Nikola Tesla had envisioned a way of transmitting electricity wirelessly. In order to understand electricity, an understanding of vibration and physics is needed. | ||
| + | |||
| + | ==Personal Project Report: Electrolysis and ICE== | ||
| + | '''What is electrolysis?''' | ||
| + | |||
| + | Electrolysis is an experiment done to observe the changes in electron behavior due to current change. Essentially, electrolysis is used to separate or decompose the electrons of a compound molecule. In this experiment, we use electrolysis to separate water and sodium bicarbonate, in hopes that we can obtain hydrogen gas out of electrolysis and try to analyze how it is implemented in an ICE engine. | ||
| + | |||
| + | '''Materials and Apparatus''' | ||
| + | |||
| + | For this project, what we'll need is: | ||
| + | Materials: | ||
| + | 1. Aluminum plates | ||
| + | 2. Container box (15 L) | ||
| + | 3. Drafts | ||
| + | 4. Used water bottles | ||
| + | 5. Garden hoses | ||
| + | 6. Sodium bicarbonate | ||
| + | 7. Balloons | ||
| + | |||
| + | Apparatus: | ||
| + | 1. Drill | ||
| + | 2. Cutters | ||
| + | 3. Beaker glass | ||
| + | 4. Power supply (12V/5A and 12V/2A) | ||
| + | 5. Cathode and anode connectors | ||
| + | |||
| + | '''Procedures''' | ||
| + | 1. Drill holes on the box container to accommodate pipes, drafts, and a cathode cover. | ||
| + | 2. Glue pipes, drafts, and cathode cover into the container | ||
| + | 3. Insert cathode steels and anode steels into the drafts | ||
| + | 4. Pour the container with water, and mix with sodium bicarbonate | ||
| + | 5. Using a 12V/5A power supply, connect the power lines into cathode and anode poles | ||
| + | 6. Insert a balloon into the cathode pipe. This will store the hydrogen | ||
| + | 7. Turn on the power, and wait 30 minutes for hydrogen to form | ||
| + | |||
| + | '''Results''' | ||
| + | From the results we can see that the more amperes are put into the system, the more power is produced. | ||
| + | |||
| + | |||
| + | == ECS Final Report == | ||
| + | For the final project, me together with Darrent made a simple electrolysis setup. The purpose of this electrolysis setup is to produce hydrogen gas using water and electricity. Using a sodium bicarbonate solution, hydrogen is extracted resulting in Hydrogen and Oxygen gas. For the conductors, plates made out of stainless steel and aluminum is used. Hydrogen is then generated in the cathode which is the + side, and oxygen at the anode which is the - side. | ||
| + | |||
| + | For the setup, we used several materials. The main container consists of a covered plastic box that is drilled on the sides and the top. The side holes is to fix the metal rod in place, and the top hole is used to make exhausts that is connected to the garden hoses. Then, the hole above the cathode side is connected to a mineral water bottle using the garden hose, and the anode side is an exhaust hose for the produced oxygen. Even though it seemed easy, the process of fixing the metal rod in place was problematic. The problem we faced that was even though a glue gun was used, the container would leak water through the metal rod. Thus, the gluing had to be done several times. The top hole for also had to be glued with the hose to ensure a leakproof connection. After fixing the rod in place, the steel plates are mounted to the steel rod. Then, the cathode side is connected with the + electricity and the anode with the -. Then, the electricity can be connected to the outlet. | ||
| + | |||
| + | For this experiment, a solution of Sodium Bicarbonate is used. A solution of Natrium Chloride can also be used, but the resulting gas would be too dangerous because it produces Chlorine gas as a byproduct. When Sodium Bicarbonate is used, a byproduct of Oxygen will be produced. | ||
| + | |||
| + | After setting up the kit, we can then turn on the DC power source. When the cathode and anode got energized, it was observed that bubbles started forming around the plates. This indicated the electrolysis is working and hydrogen is being produced. The hydrogen is then stored in the mineral water bottle, where then it can be used for whatever purpose. | ||
| + | |||
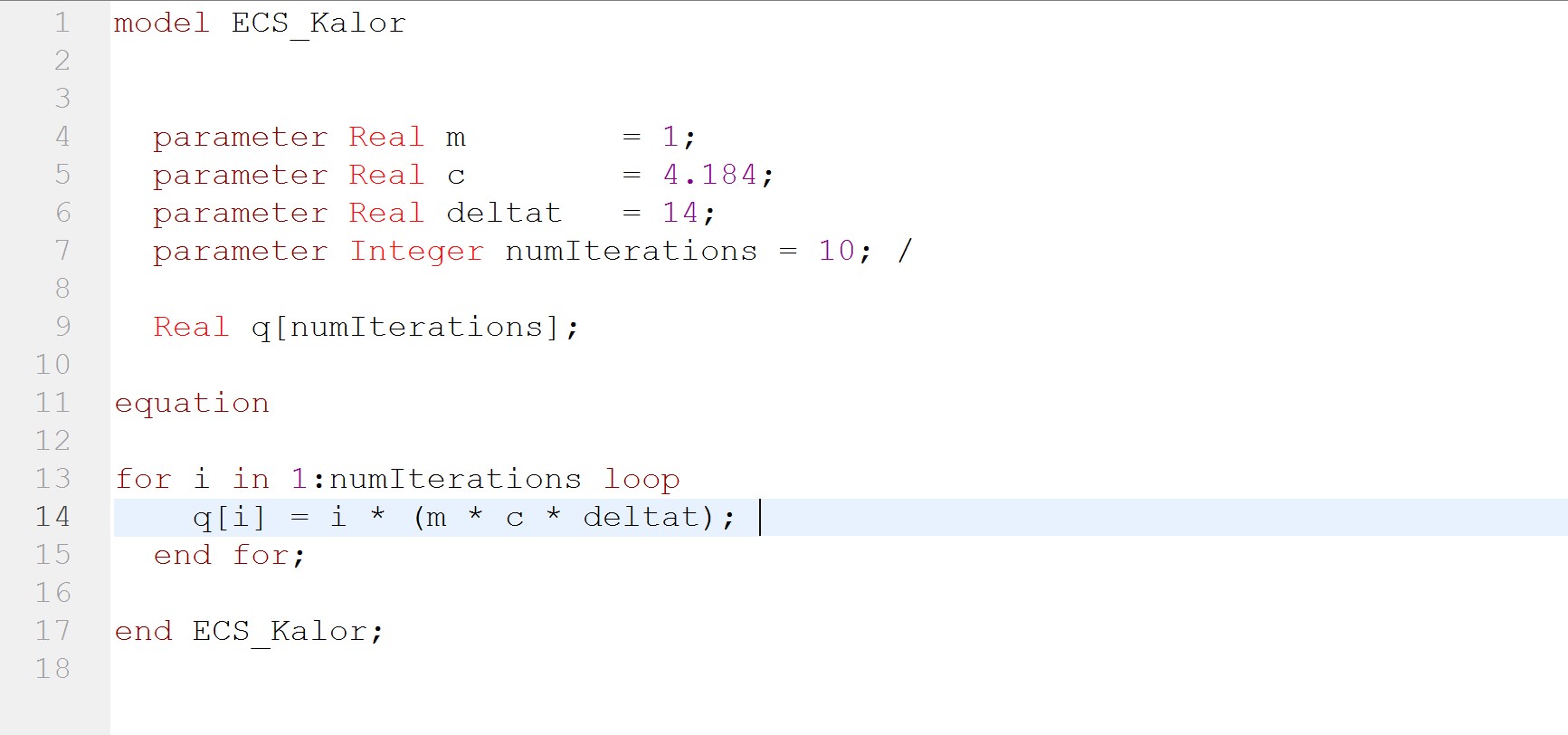
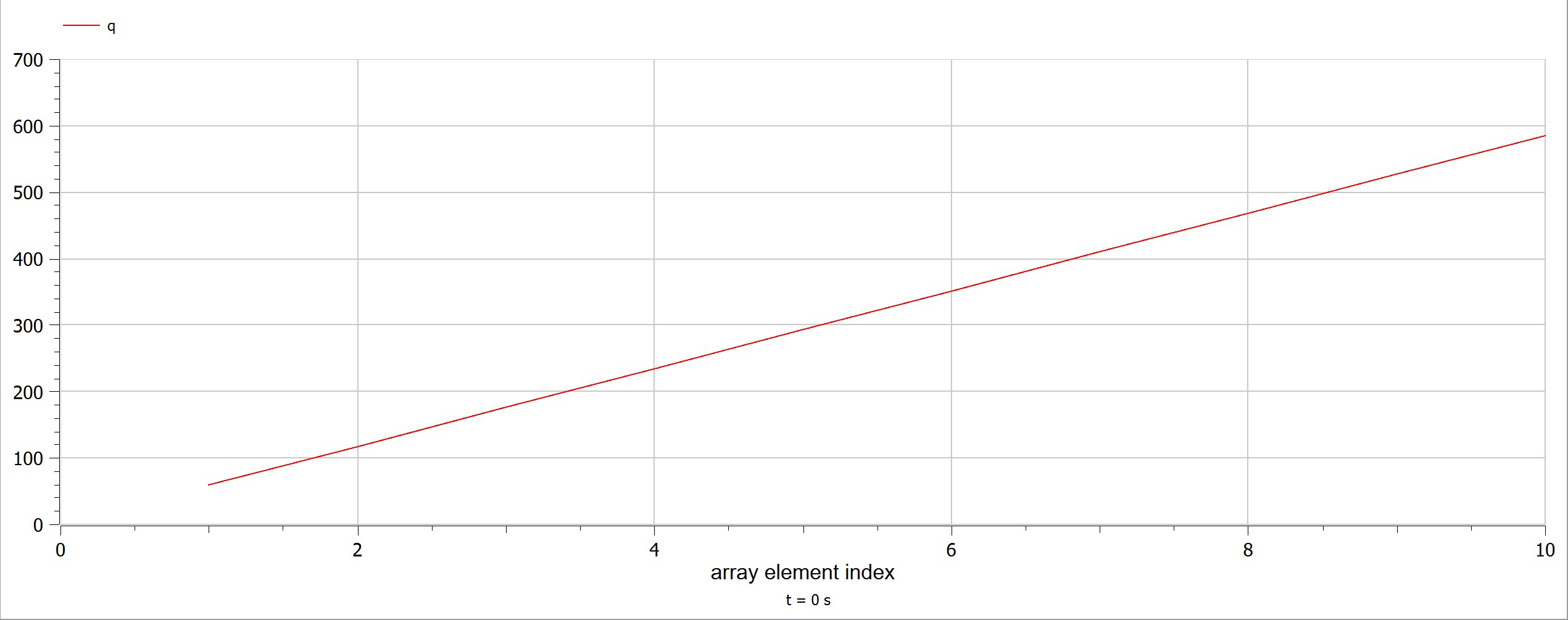
| + | The experiment also had some problems, such as the data as the electricity source was too small and hence it was a little bit difficult to obtain data. Using limited electricity, only a small amount of hydrogen can be obtained/produced. [[File:1231.jpg]] | ||
| + | OpenModelica Model | ||
| + | [[File:1232.jpg]] | ||
| + | [[File:1233.jpg]] | ||
Latest revision as of 10:26, 13 June 2023
hi, welcome to my page.
Full name : Ignatius Adrian
Student Number : 2006606561
Tuesday, 21 February 2023
ChatGPT : If we use sugar cane to generate energy instead of petroleum and natural gas, then we would be reducing our dependence on fossil fuels, which would reduce greenhouse gas emissions. This would help to address climate change. Additionally, sugar cane is a renewable resource, so it can be grown in perpetuity, which is more sustainable than fossil fuels. Finally, by using sugar cane, we would be supporting local farmers and reducing reliance on foreign countries for energy resources. So sugar cane is the preferred choice for many reasons.
The relation between all three of these businesses is that they all use resources.
Oil and gas companies supply oil and gas to power the automotive factories.
The automotive factories produce cars that transport goods.
And sugar cane factories turn raw sugar cane into raw sugar, so that food companies can make food products and sell them everywhere.
The oil and gas companies use the automotive factories to transport resources. And the automotive factories deliver the resources to the sugar cane factories.
The automotive factories need the natural gas and gasoline of the oil and gas companies to create vehicles.
Contents
Friday, 24 February 2023
Today, we learned how to create an alternating engine (internal combustion engine) using OpenModelica. Before creating the model, we had to install the ThermosysPro library, only after that will we be able to create the model. After installing the library, we opened a new model file and proceeded to import the Alternating Engine model from the library we just downloaded.
After importing, we then added the Fuel Source PQ and Source PQ to the engine. The Fuel Source PQ is the fuel source, which is gasoline, and the source PQ is the air that is oxygen.
Fuel Source PQ is connected to the left side, which is the inlet of the internal combustion engine. Next, source PQ is connected to air inlet for water cooling and air inlet. Then, seek is connected to the right side of the engine that is the outlet.
Next, we add singular pressure loss between Source PQ and cooling inlet, and between cooling outlet and seek.
Using the reference from CCIT, we can then use the variables off of the reference.
Project Proposal
For my personal project, I propose that we switch to electric-powered cargo ships. This idea falls into the EV Category. Cargo ships contributes 3.5% to 4% of all climate change emissions, mostly carbon dioxide. The use of electric-powered cargo ships should be able to reduce global warming.
The World Bank stated that, cargo ships are the 6th biggest contributor towards the world's carbon emission. This is because cargo ships operate near 24/7, produces a lot of carbon dioxide because of the fuel consumed in transport, and the sheer amount of existing cargo ships. The use of electric power as a substitute will bring great benefits to our planet. An electric cargo ship can be powered by a 6.8MWH battery, which is approximately a hundred times the size of an electric car's battery. The power is mainly harvested off hydro power. Using an average cargo ship's dimensions and weight, I can then model a cargo ship using a battery on OpenModelica and compare the emissions generated by a fully electric cargo ship.
Tuesday, 28 February 2023
Today, Professor Adi explained to us regarding ICE, Pyrolysis, and EV. We talked about how combustion occurs, how it requires oxygen and carbon, and as a byproduct CO2 and water is produced. Pyrolysis is the process in which waste is burned and converted into biofuel. The waste which can be converted into biofuel are organic materials such as biomass. Combustion and pyrolysis is based off the process that is the ignition of carbon.
Mr DAI told us that Nikola Tesla had envisioned a way of transmitting electricity wirelessly. In order to understand electricity, an understanding of vibration and physics is needed.
Personal Project Report: Electrolysis and ICE
What is electrolysis?
Electrolysis is an experiment done to observe the changes in electron behavior due to current change. Essentially, electrolysis is used to separate or decompose the electrons of a compound molecule. In this experiment, we use electrolysis to separate water and sodium bicarbonate, in hopes that we can obtain hydrogen gas out of electrolysis and try to analyze how it is implemented in an ICE engine.
Materials and Apparatus
For this project, what we'll need is: Materials: 1. Aluminum plates 2. Container box (15 L) 3. Drafts 4. Used water bottles 5. Garden hoses 6. Sodium bicarbonate 7. Balloons
Apparatus: 1. Drill 2. Cutters 3. Beaker glass 4. Power supply (12V/5A and 12V/2A) 5. Cathode and anode connectors
Procedures 1. Drill holes on the box container to accommodate pipes, drafts, and a cathode cover. 2. Glue pipes, drafts, and cathode cover into the container 3. Insert cathode steels and anode steels into the drafts 4. Pour the container with water, and mix with sodium bicarbonate 5. Using a 12V/5A power supply, connect the power lines into cathode and anode poles 6. Insert a balloon into the cathode pipe. This will store the hydrogen 7. Turn on the power, and wait 30 minutes for hydrogen to form
Results From the results we can see that the more amperes are put into the system, the more power is produced.
ECS Final Report
For the final project, me together with Darrent made a simple electrolysis setup. The purpose of this electrolysis setup is to produce hydrogen gas using water and electricity. Using a sodium bicarbonate solution, hydrogen is extracted resulting in Hydrogen and Oxygen gas. For the conductors, plates made out of stainless steel and aluminum is used. Hydrogen is then generated in the cathode which is the + side, and oxygen at the anode which is the - side.
For the setup, we used several materials. The main container consists of a covered plastic box that is drilled on the sides and the top. The side holes is to fix the metal rod in place, and the top hole is used to make exhausts that is connected to the garden hoses. Then, the hole above the cathode side is connected to a mineral water bottle using the garden hose, and the anode side is an exhaust hose for the produced oxygen. Even though it seemed easy, the process of fixing the metal rod in place was problematic. The problem we faced that was even though a glue gun was used, the container would leak water through the metal rod. Thus, the gluing had to be done several times. The top hole for also had to be glued with the hose to ensure a leakproof connection. After fixing the rod in place, the steel plates are mounted to the steel rod. Then, the cathode side is connected with the + electricity and the anode with the -. Then, the electricity can be connected to the outlet.
For this experiment, a solution of Sodium Bicarbonate is used. A solution of Natrium Chloride can also be used, but the resulting gas would be too dangerous because it produces Chlorine gas as a byproduct. When Sodium Bicarbonate is used, a byproduct of Oxygen will be produced.
After setting up the kit, we can then turn on the DC power source. When the cathode and anode got energized, it was observed that bubbles started forming around the plates. This indicated the electrolysis is working and hydrogen is being produced. The hydrogen is then stored in the mineral water bottle, where then it can be used for whatever purpose.
The experiment also had some problems, such as the data as the electricity source was too small and hence it was a little bit difficult to obtain data. Using limited electricity, only a small amount of hydrogen can be obtained/produced. 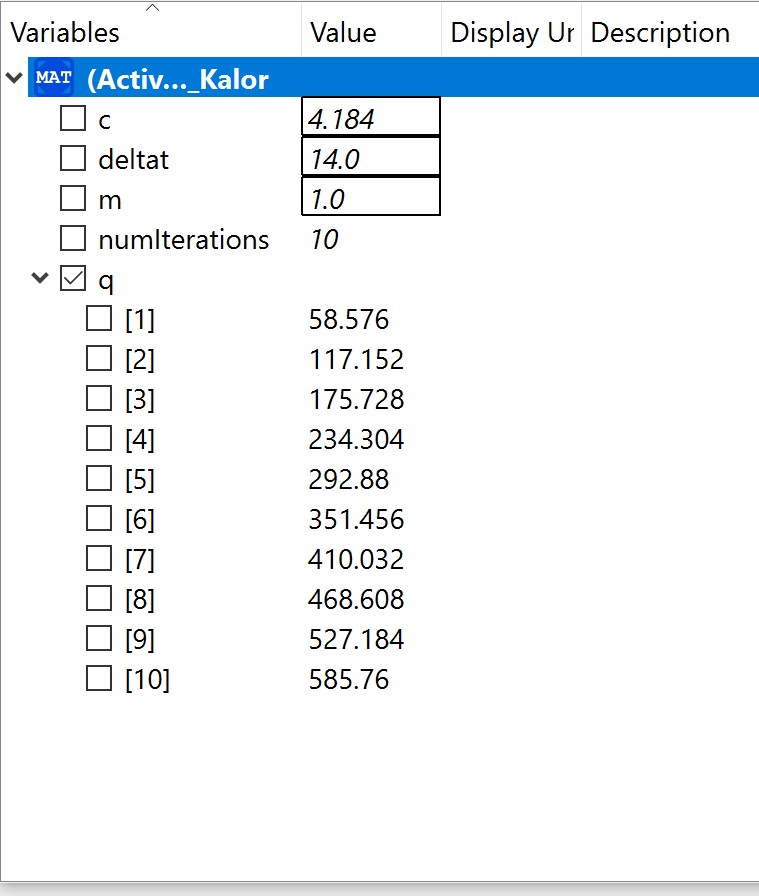 OpenModelica Model
OpenModelica Model