Difference between revisions of "Muhammad Alif Luthfi"
| Line 53: | Line 53: | ||
'''I. Basic Principle''' | '''I. Basic Principle''' | ||
| − | [[File:DipsH2O2reaction. | + | [[File:DipsH2O2reaction.png]] |
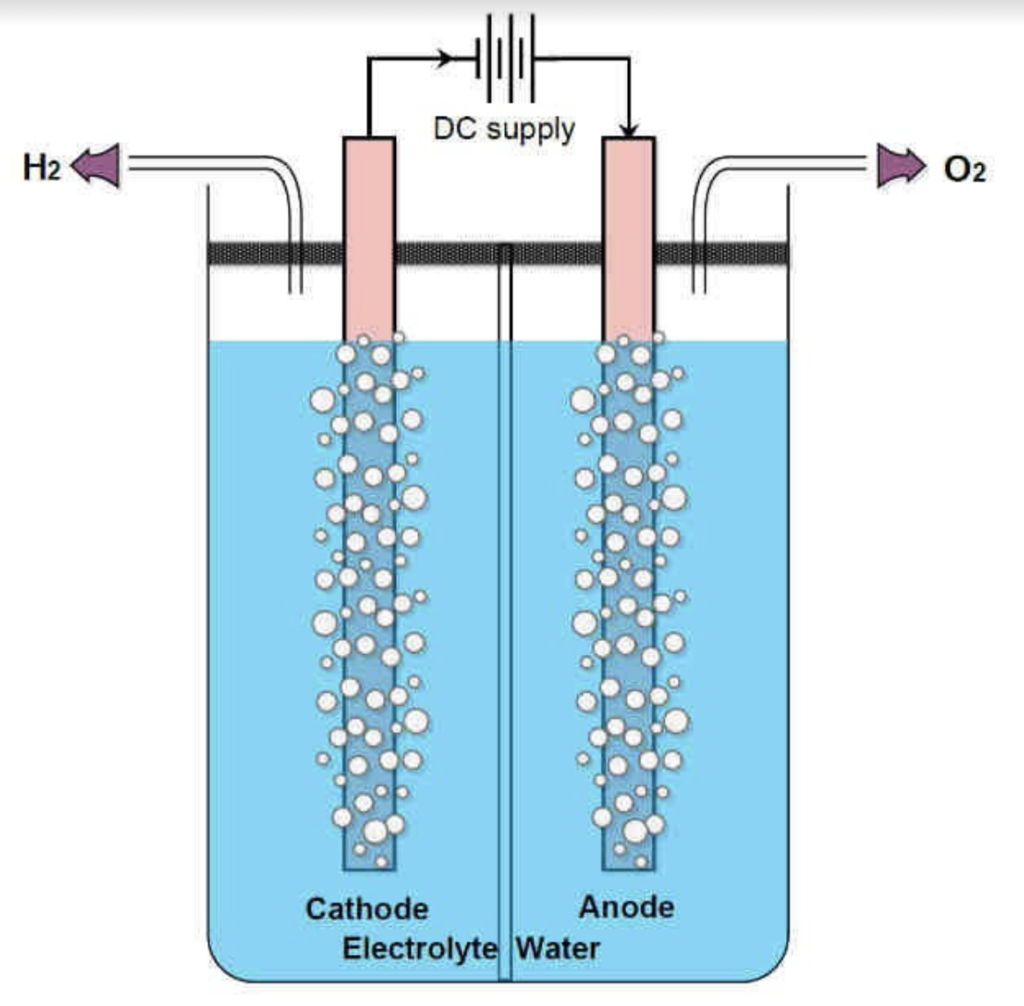
Free hydrogen and oxygen molecules readily react with each other to produce water. At a low auto-ignition temperature of 570 C, along with its highly exothermic reaction, it became a potential alternative fuel for future vehicles. Hydrogen is mass-produced from natural gas, however, the production of hydrogen using this method also produces a byproduct of carbon dioxide, which negates the benefit of its usage as an alternate fuel. ''Green'' hydrogen, or hydrogen that is produced without the production of its byproduct is possible. Using a method called electrolysis, hydrogen can be produced from water. | Free hydrogen and oxygen molecules readily react with each other to produce water. At a low auto-ignition temperature of 570 C, along with its highly exothermic reaction, it became a potential alternative fuel for future vehicles. Hydrogen is mass-produced from natural gas, however, the production of hydrogen using this method also produces a byproduct of carbon dioxide, which negates the benefit of its usage as an alternate fuel. ''Green'' hydrogen, or hydrogen that is produced without the production of its byproduct is possible. Using a method called electrolysis, hydrogen can be produced from water. | ||
| − | [[File:DipsElectrolysis. | + | [[File:DipsElectrolysis.png]] |
This process takes place in an electrolyzer, which consists of two electrodes and an electrolyte. When current flows through the electrolyte (commonly water), the water molecules in the anode are oxidized and produce oxygen while the molecules in the cathode are reduced and produce hydrogen. | This process takes place in an electrolyzer, which consists of two electrodes and an electrolyte. When current flows through the electrolyte (commonly water), the water molecules in the anode are oxidized and produce oxygen while the molecules in the cathode are reduced and produce hydrogen. | ||
| Line 63: | Line 63: | ||
'''II. Experiment Setup''' | '''II. Experiment Setup''' | ||
| − | [[File: | + | [[File:DipsPlate.jpg]] |
I reproduced this phenomenon using 8 plates of 1 mm thick aluminum 6061 plates. Then, I tested this setup on Rasen's electrolyzer. The electrolyte is mixed with KOH and the electrodes are supplied with a switching DC power supply. | I reproduced this phenomenon using 8 plates of 1 mm thick aluminum 6061 plates. Then, I tested this setup on Rasen's electrolyzer. The electrolyte is mixed with KOH and the electrodes are supplied with a switching DC power supply. | ||
Revision as of 05:24, 11 April 2023
Hello everyone, this page will be the wiki for my Energy Conversion System 2 Class.
Contents
The connection between the sugar cane industry, battery industry, and oil and gas industry.
Me: Are there any connection between the sugar cane industry, battery industry, and oil and gas industry
ChatGPT: Yes, there are connections between the sugar cane industry, battery industry, and oil and gas industry.
One connection between the sugar cane industry and the battery industry is through the production of biofuels. Sugar cane can be used to produce ethanol, which is a biofuel that can be blended with gasoline to reduce greenhouse gas emissions. Ethanol can also be used as a fuel for fuel cells, which are used to power electric vehicles. Additionally, some types of batteries, such as flow batteries, can use sugars as an electrolyte.
Another connection between the sugar cane industry and the oil and gas industry is through the production of bioplastics. Bioplastics can be made from sugar cane or other plant-based materials, which can reduce the dependence on fossil fuels for plastic production. However, some bioplastics still require oil and gas derivatives in their manufacturing process.
The connection between the battery industry and the oil and gas industry is through the production of lithium-ion batteries, which are commonly used in electric vehicles and other electronic devices. Lithium is a rare metal that is typically mined from the earth, and some of the largest lithium deposits are found in areas that also produce oil and gas, such as the lithium triangle in South America. Additionally, the production and disposal of batteries can have environmental impacts, which are often linked to the production and consumption of fossil fuels.
Overall, while there are connections between these industries, efforts are being made to reduce the dependence on fossil fuels and minimize the environmental impacts of their operations.
Internal Combustion Engine Personal Project
Title: Ammonia as an alternative fuel for Internal Combustion Engine (ICE)
Ammonia (NH3) is a lesser-known alternative fuel for ICEs. It is very similar to Hydrogen, in that they do not produce greenhouse gas when burned. It also brings additional benefits in the form of ease of storage. However, in terms of performance, how does it fare against conventional gasoline and hydrogen fuels? Also, What drawbacks does it bring?. In this project, I will try to answer these questions.
Simulation of Internal Combustion Engine using OpenModelica
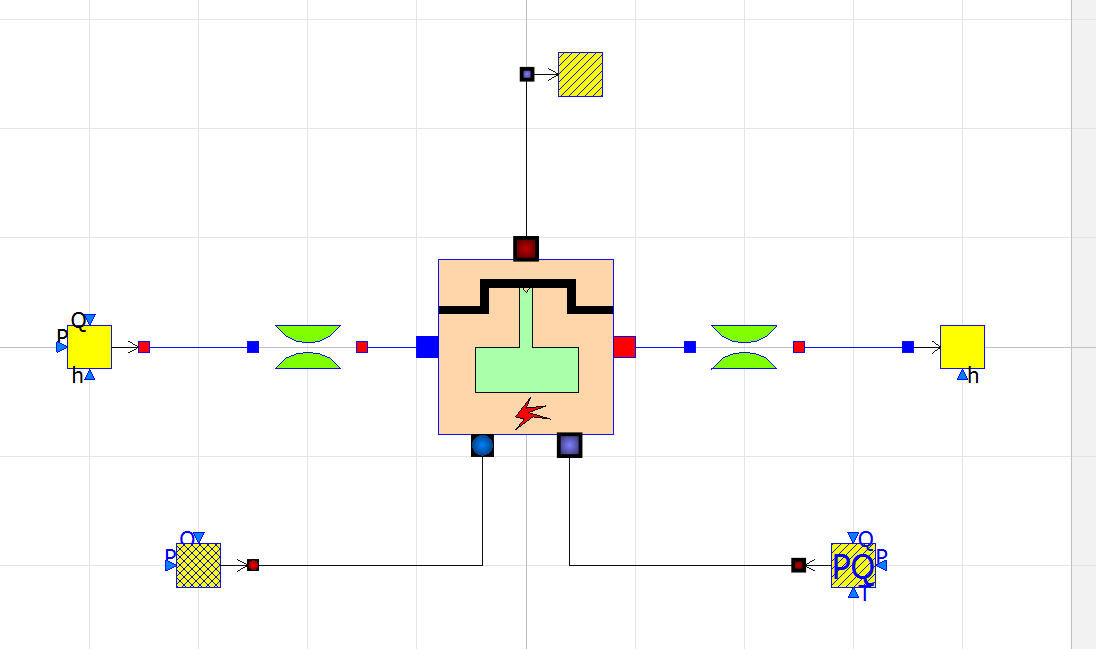
There are 7 components that governs the simulation of an Internal Combustion Engine in OpenModelica
- 1. alternatingEngine
ThermoSysPro.MultiFluids.Machines.AlternatingEngine
- This is the main component in the model, this component solves the equations for Internal Combustion Engine to obtain the power output of the engine.
- 2. fuelSourcePQ
ThermoSysPro.Combustion.BoundaryConditions.FuelSourcePQ
- This is the fuel source of the engine, parameters such as the fuel composition and flow rate can be changed.
- 3. sourceAir
ThermoSysPro.FlueGases.BoundaryConditions.SourcePQ
- This is the air source of the engine, parameters such as the air pressure and flow rate can be changed.
- 4. sink
ThermoSysPro.FlueGases.BoundaryConditions.Sink
- This is the exhaust of the engine (sink).
- 5. SourcePQ_Water
ThermoSysPro.WaterSteam.BoundaryConditions.SourcePQ
- This is the water cooling system inlet of the engine, the enthalpy and flow rate of the coolant can be changed.
- 6. Sink_Water
ThermoSysPro.WaterSteam.BoundaryConditions.Sink
- This is the water cooling system outlet of the engine.
- 7. singularPressureLoss
ThermoSysPro.WaterSteam.PressureLosses.SingularPressureLoss
- This component simulates the pressure loss within the cooling system.
HHO Generator Project
With oil prices constantly rising, many are trying to find a way to minimize their spending on gas stations. One such method is by using an HHO Generator. Nowadays, many HHO generators are being sold online, claiming that they can improve your car mileage by 35%. However, are these claims true? or are they just lies spread to make people buy those products? If you ever watched The Mythbusters, you probably already know the answer.
I. Basic Principle
Free hydrogen and oxygen molecules readily react with each other to produce water. At a low auto-ignition temperature of 570 C, along with its highly exothermic reaction, it became a potential alternative fuel for future vehicles. Hydrogen is mass-produced from natural gas, however, the production of hydrogen using this method also produces a byproduct of carbon dioxide, which negates the benefit of its usage as an alternate fuel. Green hydrogen, or hydrogen that is produced without the production of its byproduct is possible. Using a method called electrolysis, hydrogen can be produced from water.
This process takes place in an electrolyzer, which consists of two electrodes and an electrolyte. When current flows through the electrolyte (commonly water), the water molecules in the anode are oxidized and produce oxygen while the molecules in the cathode are reduced and produce hydrogen.
II. Experiment Setup
I reproduced this phenomenon using 8 plates of 1 mm thick aluminum 6061 plates. Then, I tested this setup on Rasen's electrolyzer. The electrolyte is mixed with KOH and the electrodes are supplied with a switching DC power supply.
The experiment is repeated three times, at varying amperages. I chose the 10 A, 7 A, and 4 A as my independent variables.
III. Result
The following are the results of the experiment.
IV. Data Processing
The results are processed using Microsoft Excel
From these data, the mass flow rate can be entered into OpenModelica to find out how much power does the hydrogen able to produce using internal combustion.
V. Conclusion
The conclusion will be revealed in my full report.