Difference between revisions of "Muhammad Dhia Andriyanto"
(→Project Progress) |
(→Hydrogen Electrolysis Power Efficiency Experiment Final Report) |
||
| (25 intermediate revisions by the same user not shown) | |||
| Line 37: | Line 37: | ||
The future for water purification/filtration is using Graphene. In the laboratory, graphene oxide membranes have been shown to be extremely effective at removing contaminants from water. Today's graphene membrane research is bringing the possibility of providing clean, safe drinking water to human being. | The future for water purification/filtration is using Graphene. In the laboratory, graphene oxide membranes have been shown to be extremely effective at removing contaminants from water. Today's graphene membrane research is bringing the possibility of providing clean, safe drinking water to human being. | ||
| + | |||
| + | |||
| + | == Quiz == | ||
| + | |||
| + | [[File:249980.jpg|300px]] | ||
== Personal Project == | == Personal Project == | ||
| Line 78: | Line 83: | ||
https://youtu.be/yG5AZMPBE14 | https://youtu.be/yG5AZMPBE14 | ||
| + | Update, I got 5 new metal plates of aluminium zinc alloy plate with 0.3mm thickness 80 x 150mm | ||
| + | |||
| + | == Electrolysis Project Report == | ||
| Line 85: | Line 93: | ||
'''II. Literature Study''' | '''II. Literature Study''' | ||
| + | |||
| + | [[File:Electrolysis-process.png]] | ||
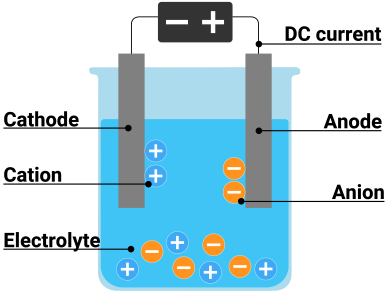
Electrolysis is a promising method for producing carbon-free hydrogen from renewable and nuclear resources. The process of splitting water into hydrogen and oxygen using electricity is known as electrolysis. This reaction takes place in a device known as an electrolyzer. Electrolyzers can range in size from small, appliance-sized equipment suitable for small-scale distributed hydrogen production to large-scale, central production facilities that could be directly linked to renewable or other non-greenhouse-gas-emitting forms of electricity generation. | Electrolysis is a promising method for producing carbon-free hydrogen from renewable and nuclear resources. The process of splitting water into hydrogen and oxygen using electricity is known as electrolysis. This reaction takes place in a device known as an electrolyzer. Electrolyzers can range in size from small, appliance-sized equipment suitable for small-scale distributed hydrogen production to large-scale, central production facilities that could be directly linked to renewable or other non-greenhouse-gas-emitting forms of electricity generation. | ||
| + | Electrolysis involves the exchange of ions and atoms caused by the addition or removal of electrons from the external circuit. In general, when current flows, cations move to the cathode, take electrons from the cathode (provided by the supply source-battery), and discharge into the neutral atom. If the neutral atom is solid, it is deposited on the cathode; if it is gas, it moves upwards. The cation is reduced at the cathode during this reduction process. At the anode, water reacts to produce oxygen and positively charged hydrogen ions. (protons). Hydrogen ions combine with electrons from the external circuit at the cathode to form hydrogen gas. Reaction at the anode: 2H2O O2 + 4H+ + 4e- & Cathode Reaction 4H+ + 4e- 2H2 | ||
| + | |||
| + | |||
| + | '''III. Equipment & Procedure''' | ||
| + | |||
| + | Equipment: | ||
| + | |||
| + | 1. Metal Plates (In my project is 0.3mm thickness of 80 x 150 mm aluminium zinc plate) | ||
| + | |||
| + | 2. Container Box | ||
| + | |||
| + | 3. hoses/Pipe | ||
| + | |||
| + | 4. Sodium Bicarbonate | ||
| + | |||
| + | 5. DC Power Supply | ||
| + | |||
| + | 6. Beaker Glass | ||
| + | |||
| + | 7. Cathode anode connectors | ||
| + | |||
| + | |||
| + | Procedure: | ||
| + | |||
| + | |||
| + | [[File:249885.jpg|300px|center]] | ||
| + | 1. Prepared the 5 Metal plates (In my project is 0.3mm thickness of 80 x 150 mm aluminium zinc plate). | ||
| + | |||
| + | [[File:249839.jpg|300px|center]] | ||
| + | |||
| + | 2. Stack the metal plates and tested on Rasendriya's electrolyzer. In this experiment, the current (A) variables are 6 A, 8 A, 10 A. | ||
| + | |||
| + | 3. Analyze & calculate the result | ||
| + | |||
| + | |||
| + | '''IV. Result''' | ||
| + | |||
| + | [[File:6a.jpg|200px|center]] [[File:6adc.jpg|200px|center]] | ||
| + | |||
| + | 6 Ampere Result = 40 ml/min | ||
| + | |||
| + | [[File:8a.jpg|200px|center]] [[File:8adc.jpg|200px|center]] | ||
| + | |||
| + | 8 Ampere Result = 45 ml/min | ||
| + | |||
| + | [[File:10a.jpg|200px|center]] [[File:10adc.jpg|200px|center]] | ||
| + | |||
| + | 10 Ampere Result = 55 ml/min | ||
| + | |||
| + | |||
| + | '''V. Calculation & Data Processing''' | ||
| + | |||
| + | [[File:MessageImage 1681186165529.jpg|800px|center]] | ||
| + | |||
| + | '''VI. Modelling & Simulation''' | ||
| + | |||
| + | |||
| + | |||
| + | '''VII. Analysis''' | ||
| + | |||
| + | |||
| + | |||
| + | '''VIII. Conclusion''' | ||
| + | |||
| + | |||
| + | For presentation mode, click this link [https://docs.google.com/presentation/d/1wHnzRVTwSQi-hMQmSoMYCSIiSzjPf0p2jAtMdLfrl1c/edit?usp=sharing] | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Hydrogen Electrolysis Power Efficiency Experiment Final Report == | ||
| + | |||
| + | |||
| + | '''Abstract''' | ||
| + | |||
| + | An experimental research into the power efficiency of hydrogen electrolysis is presented in this work. Hydrogen electrolysis is a promising approach for creating hydrogen as an environmentally friendly and sustainable energy carrier. However, several factors like electrode materials, electrolyte composition, and operating circumstances influence the overall efficiency of the process. A systematic examination of power efficiency was performed in this study by altering these parameters in a laboratory-scale electrolysis cell. The findings show that electrode material has a substantial impact on power consumption and hydrogen production rate. The effects of electrolyte content and operation temperature on efficiency were also investigated. The findings shed light on how to improve the power efficiency of hydrogen electrolysis, which is critical for expanding the commercial feasibility of this technology in a sustainable manner. | ||
| + | |||
| + | |||
| + | '''I. Introduction''' | ||
| + | |||
| + | As a clean and adaptable energy carrier, hydrogen has emerged as a possible solution to the challenges of achieving a sustainable energy future. Its prospective uses in sectors including transportation, electricity generation, and industrial processes have sparked considerable interest around the world. Water electrolysis stands out as a major method of hydrogen generation due to its potential to generate hydrogen from renewable energy sources such as solar and wind. | ||
| + | The process of splitting water molecules (H2O) into their basic elements, hydrogen (H2) and oxygen (O2), is known as electrolysis. This chemical process takes place in an electrolyzer, which is made up of two electrodes immersed in an electrolyte solution. When a direct current is provided, the cathode attracts and reduces positively charged hydrogen ions (H+) to make hydrogen gas (H2), while the anode attracts and oxidizes negatively charged hydroxide ions (OH-) to produce oxygen gas (O2). | ||
| + | This paper aims to investigate the power efficiency of hydrogen electrolysis through a comprehensive experimental approach. By systematically varying key parameters such as current density, electrode materials, and electrolyte composition, we seek to understand the factors that influence the efficiency of the electrolysis process. Furthermore, we aim to explore the potential of optimizing these parameters to enhance power efficiency and contribute to the development of more efficient and sustainable hydrogen production systems. | ||
| + | The findings from this research have significant implications for the design and optimization of electrolysis systems. By identifying the factors that influence power efficiency, we can provide insights to guide the development of advanced electrolyzer technologies, inform policy decisions regarding hydrogen production, and contribute to the broader goal of achieving a carbon-neutral and sustainable energy ecosystem. | ||
| + | |||
| + | |||
| + | '''II. Objectives''' | ||
| + | |||
| + | 1. To investigate the power efficiency of hydrogen electrolysis as a method for hydrogen production. | ||
| + | |||
| + | 2. To determine the optimal conditions and parameters for achieving high power efficiency in hydrogen electrolysis. | ||
| + | |||
| + | 3. To design models of combustion in OpenModelica | ||
| + | |||
| + | |||
| + | |||
| + | '''III. Preliminary Question''' | ||
| + | |||
| + | 1. How does the electrolyte concentration affect the power efficiency of hydrogen electrolysis? | ||
| + | |||
| + | 2. How does the temperature of the electrolyte solution influence power efficiency? | ||
| + | |||
| + | 3. What role does the applied voltage or current density play in determining power efficiency? | ||
| + | |||
| + | 4. What factors influence the power efficiency of hydrogen electrolysis? | ||
| + | |||
| + | |||
| + | |||
| + | '''IV. Theory and Discussion''' | ||
| + | |||
| + | Hydrogen electrolysis is the use of an electric current to split water molecules into hydrogen and oxygen gases. The procedure takes place in an electrolyzer, which is made up of two electrodes submerged in an electrolyte solution. The anode draws negatively charged oxygen ions (O2-) while the cathode attracts positively charged hydrogen ions (H+). The electrolyzer's electric current encourages ion migration, resulting in the creation of hydrogen gas at the cathode and oxygen gas at the anode. The energy required to generate a certain amount of hydrogen gas determines power efficiency in hydrogen electrolysis. | ||
| + | The power efficiency of hydrogen electrolysis experiments is influenced by a number of factors. The cell voltage, or the applied potential difference across the electrolyzer, is an important aspect. Reduced energy losses during the electrolysis process result in improved power efficiency with lower cell voltage. Furthermore, the electrolyte used has a considerable impact on power efficiency. Low resistance conductive electrolytes enable for more efficient ion migration, resulting in increased power efficiency. Furthermore, the design and materials of the electrodes have an impact on total efficiency. High-quality electrodes with wide surface areas and high catalytic characteristics can boost hydrogen evolution while reducing energy losses. | ||
| + | Several ways can be used to improve the power efficiency of hydrogen electrolysis experiments. To begin, optimizing the electrolyzer design is critical. Increasing the surface area of the electrodes can allow for a higher rate of hydrogen synthesis, resulting in increased efficiency. Furthermore, the use of sophisticated catalyst materials, such as platinum, can improve the kinetics of the hydrogen evolution reaction, lowering energy needs. Second, using proton exchange membrane (PEM) electrolyzers can improve power efficiency. PEM electrolyzers provide lower cell voltages and improved control over the electrolysis process, resulting in higher efficiency. Finally, incorporating renewable energy sources, such as solar or wind power, to provide the electric current for electrolysis can considerably increase the process's sustainability and overall efficiency. | ||
| + | While hydrogen electrolysis has enormous promise for long-term energy production, various hurdles must be overcome in order to improve its power efficiency even more. The high cost of catalyst materials is a major impediment to large-scale implementation. To overcome this barrier, research efforts should focus on producing low-cost and efficient catalysts. Scaling up electrolysis systems without sacrificing efficiency is also an issue. Engineering breakthroughs and economies of scale can help to solve this problem. Furthermore, investigating novel electrolyte solutions and electrode materials with increased ion conductivity and catalytic activity could improve power efficiency even further. | ||
| + | In hydrogen electrolysis experiments, power efficiency is an important characteristic to consider. Understanding the elements that influence efficiency and putting improvement methods in place are critical for the widespread adoption of this technology. Power efficiency can be considerably improved by optimizing electrolyzer design, using modern catalyst materials, using PEM electrolyzers, and incorporating renewable energy sources. However, resolving cost, scalability, and material development problems is critical for future breakthroughs in hydrogen electrolysis power efficiency. | ||
| + | |||
| + | '''V. Equipment & Procedure''' | ||
| + | |||
| + | a. Equipment | ||
| + | |||
| + | 1. Metal Plates (In my project is 0.3mm thickness of 80x150mm aluminium zinc plate) | ||
| + | |||
| + | 2. Container Box | ||
| + | |||
| + | 3. hoses/Pipe | ||
| + | |||
| + | 4. Sodium Bicarbonate | ||
| + | |||
| + | 5. DC Power Supply | ||
| + | |||
| + | 6. Beaker Glass/plastic bottle | ||
| + | |||
| + | 7. Cathode anode connectors | ||
| + | |||
| + | 8. One way valve | ||
| + | |||
| + | 9. Nozzle | ||
| + | |||
| + | b. Procedure | ||
| + | |||
| + | 1. Prepared the 5 Metal plates (In my project is 0.3mm thickness of 80 x 150 mm aluminium zinc plate). | ||
| + | |||
| + | 2. Stack the metal plates and tested on an electrolyzer. In this experiment, the current (A) is Low which is 5A | ||
| + | |||
| + | 3. Calculate and Analyze the result | ||
| + | |||
| + | |||
| + | |||
| + | '''VI. Result, Calculation, & Data Processing''' | ||
| + | |||
| + | |||
| + | |||
| + | '''VII. Modelling & Simulation''' | ||
| + | |||
| + | |||
| + | |||
| + | '''VIII. Analysis''' | ||
| + | |||
| + | The experiment data were examined to determine the power efficiency of the electrolysis process. The energy content of the hydrogen produced was divided by the electrical power input to calculate the power efficiency. The data revealed a direct association between the input power level and the electrolysis system's efficiency. Increased power inputs often resulted in increased hydrogen generation rates, but at the expense of decreased power efficiency. | ||
| + | The experiment also illustrated the impact of other factors on power efficiency, such as electrolyzer type and condition, temperature, and electrolyte composition. These factors can have a considerable impact on the performance and efficiency of electrolysis systems and should be taken into account when developing and optimizing hydrogen production installations. | ||
| + | While the experiment revealed useful information about the power efficiency of the hydrogen electrolysis system, many limitations should be noted. The experiment was carried out with a modest current of only 5 Amperes and without the use of a vacuum. As a result, the flow rate, temperature, and condition are all inaccurate. Because the experiment only uses one variable, the results cannot be compared to those of other variables. A more detailed investigation of the system's overall energy balance is proposed to improve the accuracy of future studies. This would entail accounting for electrical circuit power losses, thermal losses, and auxiliary power use. Exploring alternate electrolyzer designs, catalysts, and operational settings may also provide further chances to improve power efficiency. | ||
| + | |||
| + | |||
| + | '''IX. Conclusion''' | ||
| + | |||
| + | 1. Higher electrolyte concentrations can enhance the efficiency of the electrolysis process. | ||
| + | |||
| + | 2. Temperature enhances ion mobility, reduces activation energy, and improves mass transfer, leading to a more efficient electrolysis process. | ||
| + | |||
| + | 3. Power efficiency of the electrolysis process varied significantly depending on the conditions and parameters applied. | ||
| + | |||
| + | 4. Need more variable of experiment to compare and analysis. | ||
| − | + | 5. Selecting the appropriate applied voltage and current density is crucial in determining the power efficiency of hydrogen electrolysis. | |
| − | |||
| − | + | For Docs Mode, Click this link [https://docs.google.com/document/d/1xVdTKV3DerinaZfVkN--8NrK09155X9uolkiV87UDgY/edit] | |
| − | + | For Presentation Mode, Click this link [https://docs.google.com/presentation/d/1oukwr5Z5HirWcK396X_VgEGvyA_m8GuxAPi4cLnAf0Y/edit#slide=id.p4] | |
Latest revision as of 10:57, 13 June 2023
Contents
Introduction
Hello everyone, my name is Muhammad Dhia Andriyanto - 2006517796 currently this is my 6th semester in Mechanical Engineering at Universitas Indonesia. This page is my summary about Energy Conversion System 2.
Chat With ChatGPT
on 21 February 2023, my friends and I chat with ChatGPT regarding the correlation between sugar cane factory, automotive factory, and oil & gas factory. When I asked ChatGPT about this topic, ChatGPT said that the correlation is the use of ethanol and its production process.
One potential link between these factories is that they are all part of the same supply chain. The sugar cane factory, for example, may supply sugar to the automotive factory, which uses it to make ethanol for fuel. The oil and gas factory may also supply fuel to the automotive factory, and some of the ethanol may be used as a blend component for gasoline. Furthermore, the oil and gas plant may provide diesel or other fuels to power the sugar cane factory's machinery. The energy is required by the sugar cane factory to power its machinery and process the sugar cane into sugar or ethanol. Fuel is required by the automotive factory to power its assembly lines and other operations. Energy is required by the oil and gas industry to extract, refine, and transport oil and gas. These factories may also be connected in terms of electricity or heat supply, depending on their location and available energy sources.
All of these factories have the potential to have serious environmental consequences. If the sugar cane factory relies on clearing land for new plantations, it may generate waste that must be disposed of or contribute to deforestation. The automotive factory's manufacturing processes may pollute the air or water, or it may contribute to greenhouse gas emissions through the use of fossil fuels. Of course, the oil and gas factory is directly involved in the extraction and processing of fossil fuels, which have numerous environmental consequences.
Apology
On Friday, 24 February 2023...I went to class 15 minutes late and Mr. DAI, Bang Tanwir, and my friends are already in class. I feel terrible and regret for what I did. Hope for the best for the future. Thank you Mr. DAI, Bang Tanwir, and friends
Class Summary
24/02/2023
On this date, my friends and I were given a tutorial by Bang Tanwir regarding how to burns fuel and air to produce power and fuel gases on Internal Combustion Engine using OpenModelica. by using OpenModelica there were ThermosysPro library inside the application and used the ICE (alternating Engine), Fuel Source, Air source, Sink, Water, and singularPressureLoss.
28/02/2023
03/03/2023
07/03/2023
Desalination is the process of removing excess salt content from water in order to create water that can be consumed by humans, animals, or plants. Solution to the Clean Water Crisis. Desalination, which converts seawater with a high salt content that is unfit for consumption into drinkable fresh water, is one solution to the clean water availability crisis that frequently occurs in Indonesia.
By utilizing seawater and processing it as drinking water, we are also reducing the use of underground water, which is thought to be the primary cause of land subsidence in various locations, particularly in Jakarta. Unfortunately, it is difficult to find investors due to the expensive equipment for desalination.
The future for water purification/filtration is using Graphene. In the laboratory, graphene oxide membranes have been shown to be extremely effective at removing contaminants from water. Today's graphene membrane research is bringing the possibility of providing clean, safe drinking water to human being.
Quiz
Personal Project
Calculating & Modelling The Efficiency Combination Between Air and Fuel on Gokart 2-Stroke Engine For Race Purpose
I. Abstract
II. Introduction
A go-kart is a four-wheeled vehicle that lacks suspension and a differential. It is a lightweight racing vehicle that is used for racing. In comparison to other vehicles, the go-kart has a low ground clearance. Engine, wheels, steering, axle, and chassis are the components of a go-kart. The go-kart uses a 150cc engine. In this case, I used Ninja 150 Super Kips 2-Stroke Engine.
III. Preliminary Question
1. Does setting a carburetor affect engine performance?
2. What is the best setting of engine for race purpose?
3. Does External factor affect engine performance?
IV. Literature Study
A two-stroke engine is a type of internal combustion engine that completes a power cycle with two strokes of the piston up and down during one power cycle, with this power cycle being completed in one crankshaft revolution. A four-stroke engine needs four piston strokes to complete a power cycle in two crankshaft revolutions. In a two-stroke engine, the end of the combustion stroke and the start of the compression stroke occur simultaneously, as do the intake and exhaust (or scavenging) functions.
Two-stroke engines frequently have a high power-to-weight ratio, with power available in a limited range of rotational speeds known as the power band. There are fewer moving parts in two-stroke engines than in four-stroke engines.
V. Calculation
VI. Modelling & Simulation
VII. Analysis
VIII. Conclusion
Electrolysis Project Progress
On 24 March 2023, I bought a square shape of Aluminium 1100, then I cut it become round shape with 5cm of Diameter.
Update, I got 5 new metal plates of aluminium zinc alloy plate with 0.3mm thickness 80 x 150mm
Electrolysis Project Report
I. Introduction
Michael Faraday popularized the term "electrolysis" in the nineteenth century. It was a method for studying chemical reactions and obtaining pure elements. Electrolysis is now commercially important because it is widely used to separate or obtain pure elements from naturally occurring sources such as ores.
II. Literature Study
Electrolysis is a promising method for producing carbon-free hydrogen from renewable and nuclear resources. The process of splitting water into hydrogen and oxygen using electricity is known as electrolysis. This reaction takes place in a device known as an electrolyzer. Electrolyzers can range in size from small, appliance-sized equipment suitable for small-scale distributed hydrogen production to large-scale, central production facilities that could be directly linked to renewable or other non-greenhouse-gas-emitting forms of electricity generation.
Electrolysis involves the exchange of ions and atoms caused by the addition or removal of electrons from the external circuit. In general, when current flows, cations move to the cathode, take electrons from the cathode (provided by the supply source-battery), and discharge into the neutral atom. If the neutral atom is solid, it is deposited on the cathode; if it is gas, it moves upwards. The cation is reduced at the cathode during this reduction process. At the anode, water reacts to produce oxygen and positively charged hydrogen ions. (protons). Hydrogen ions combine with electrons from the external circuit at the cathode to form hydrogen gas. Reaction at the anode: 2H2O O2 + 4H+ + 4e- & Cathode Reaction 4H+ + 4e- 2H2
III. Equipment & Procedure
Equipment:
1. Metal Plates (In my project is 0.3mm thickness of 80 x 150 mm aluminium zinc plate)
2. Container Box
3. hoses/Pipe
4. Sodium Bicarbonate
5. DC Power Supply
6. Beaker Glass
7. Cathode anode connectors
Procedure:
1. Prepared the 5 Metal plates (In my project is 0.3mm thickness of 80 x 150 mm aluminium zinc plate).
2. Stack the metal plates and tested on Rasendriya's electrolyzer. In this experiment, the current (A) variables are 6 A, 8 A, 10 A.
3. Analyze & calculate the result
IV. Result
6 Ampere Result = 40 ml/min
8 Ampere Result = 45 ml/min
10 Ampere Result = 55 ml/min
V. Calculation & Data Processing
VI. Modelling & Simulation
VII. Analysis
VIII. Conclusion
For presentation mode, click this link [1]
Hydrogen Electrolysis Power Efficiency Experiment Final Report
Abstract
An experimental research into the power efficiency of hydrogen electrolysis is presented in this work. Hydrogen electrolysis is a promising approach for creating hydrogen as an environmentally friendly and sustainable energy carrier. However, several factors like electrode materials, electrolyte composition, and operating circumstances influence the overall efficiency of the process. A systematic examination of power efficiency was performed in this study by altering these parameters in a laboratory-scale electrolysis cell. The findings show that electrode material has a substantial impact on power consumption and hydrogen production rate. The effects of electrolyte content and operation temperature on efficiency were also investigated. The findings shed light on how to improve the power efficiency of hydrogen electrolysis, which is critical for expanding the commercial feasibility of this technology in a sustainable manner.
I. Introduction
As a clean and adaptable energy carrier, hydrogen has emerged as a possible solution to the challenges of achieving a sustainable energy future. Its prospective uses in sectors including transportation, electricity generation, and industrial processes have sparked considerable interest around the world. Water electrolysis stands out as a major method of hydrogen generation due to its potential to generate hydrogen from renewable energy sources such as solar and wind. The process of splitting water molecules (H2O) into their basic elements, hydrogen (H2) and oxygen (O2), is known as electrolysis. This chemical process takes place in an electrolyzer, which is made up of two electrodes immersed in an electrolyte solution. When a direct current is provided, the cathode attracts and reduces positively charged hydrogen ions (H+) to make hydrogen gas (H2), while the anode attracts and oxidizes negatively charged hydroxide ions (OH-) to produce oxygen gas (O2). This paper aims to investigate the power efficiency of hydrogen electrolysis through a comprehensive experimental approach. By systematically varying key parameters such as current density, electrode materials, and electrolyte composition, we seek to understand the factors that influence the efficiency of the electrolysis process. Furthermore, we aim to explore the potential of optimizing these parameters to enhance power efficiency and contribute to the development of more efficient and sustainable hydrogen production systems. The findings from this research have significant implications for the design and optimization of electrolysis systems. By identifying the factors that influence power efficiency, we can provide insights to guide the development of advanced electrolyzer technologies, inform policy decisions regarding hydrogen production, and contribute to the broader goal of achieving a carbon-neutral and sustainable energy ecosystem.
II. Objectives
1. To investigate the power efficiency of hydrogen electrolysis as a method for hydrogen production.
2. To determine the optimal conditions and parameters for achieving high power efficiency in hydrogen electrolysis.
3. To design models of combustion in OpenModelica
III. Preliminary Question
1. How does the electrolyte concentration affect the power efficiency of hydrogen electrolysis?
2. How does the temperature of the electrolyte solution influence power efficiency?
3. What role does the applied voltage or current density play in determining power efficiency?
4. What factors influence the power efficiency of hydrogen electrolysis?
IV. Theory and Discussion
Hydrogen electrolysis is the use of an electric current to split water molecules into hydrogen and oxygen gases. The procedure takes place in an electrolyzer, which is made up of two electrodes submerged in an electrolyte solution. The anode draws negatively charged oxygen ions (O2-) while the cathode attracts positively charged hydrogen ions (H+). The electrolyzer's electric current encourages ion migration, resulting in the creation of hydrogen gas at the cathode and oxygen gas at the anode. The energy required to generate a certain amount of hydrogen gas determines power efficiency in hydrogen electrolysis. The power efficiency of hydrogen electrolysis experiments is influenced by a number of factors. The cell voltage, or the applied potential difference across the electrolyzer, is an important aspect. Reduced energy losses during the electrolysis process result in improved power efficiency with lower cell voltage. Furthermore, the electrolyte used has a considerable impact on power efficiency. Low resistance conductive electrolytes enable for more efficient ion migration, resulting in increased power efficiency. Furthermore, the design and materials of the electrodes have an impact on total efficiency. High-quality electrodes with wide surface areas and high catalytic characteristics can boost hydrogen evolution while reducing energy losses. Several ways can be used to improve the power efficiency of hydrogen electrolysis experiments. To begin, optimizing the electrolyzer design is critical. Increasing the surface area of the electrodes can allow for a higher rate of hydrogen synthesis, resulting in increased efficiency. Furthermore, the use of sophisticated catalyst materials, such as platinum, can improve the kinetics of the hydrogen evolution reaction, lowering energy needs. Second, using proton exchange membrane (PEM) electrolyzers can improve power efficiency. PEM electrolyzers provide lower cell voltages and improved control over the electrolysis process, resulting in higher efficiency. Finally, incorporating renewable energy sources, such as solar or wind power, to provide the electric current for electrolysis can considerably increase the process's sustainability and overall efficiency. While hydrogen electrolysis has enormous promise for long-term energy production, various hurdles must be overcome in order to improve its power efficiency even more. The high cost of catalyst materials is a major impediment to large-scale implementation. To overcome this barrier, research efforts should focus on producing low-cost and efficient catalysts. Scaling up electrolysis systems without sacrificing efficiency is also an issue. Engineering breakthroughs and economies of scale can help to solve this problem. Furthermore, investigating novel electrolyte solutions and electrode materials with increased ion conductivity and catalytic activity could improve power efficiency even further. In hydrogen electrolysis experiments, power efficiency is an important characteristic to consider. Understanding the elements that influence efficiency and putting improvement methods in place are critical for the widespread adoption of this technology. Power efficiency can be considerably improved by optimizing electrolyzer design, using modern catalyst materials, using PEM electrolyzers, and incorporating renewable energy sources. However, resolving cost, scalability, and material development problems is critical for future breakthroughs in hydrogen electrolysis power efficiency.
V. Equipment & Procedure
a. Equipment
1. Metal Plates (In my project is 0.3mm thickness of 80x150mm aluminium zinc plate)
2. Container Box
3. hoses/Pipe
4. Sodium Bicarbonate
5. DC Power Supply
6. Beaker Glass/plastic bottle
7. Cathode anode connectors
8. One way valve
9. Nozzle
b. Procedure
1. Prepared the 5 Metal plates (In my project is 0.3mm thickness of 80 x 150 mm aluminium zinc plate).
2. Stack the metal plates and tested on an electrolyzer. In this experiment, the current (A) is Low which is 5A
3. Calculate and Analyze the result
VI. Result, Calculation, & Data Processing
VII. Modelling & Simulation
VIII. Analysis
The experiment data were examined to determine the power efficiency of the electrolysis process. The energy content of the hydrogen produced was divided by the electrical power input to calculate the power efficiency. The data revealed a direct association between the input power level and the electrolysis system's efficiency. Increased power inputs often resulted in increased hydrogen generation rates, but at the expense of decreased power efficiency. The experiment also illustrated the impact of other factors on power efficiency, such as electrolyzer type and condition, temperature, and electrolyte composition. These factors can have a considerable impact on the performance and efficiency of electrolysis systems and should be taken into account when developing and optimizing hydrogen production installations. While the experiment revealed useful information about the power efficiency of the hydrogen electrolysis system, many limitations should be noted. The experiment was carried out with a modest current of only 5 Amperes and without the use of a vacuum. As a result, the flow rate, temperature, and condition are all inaccurate. Because the experiment only uses one variable, the results cannot be compared to those of other variables. A more detailed investigation of the system's overall energy balance is proposed to improve the accuracy of future studies. This would entail accounting for electrical circuit power losses, thermal losses, and auxiliary power use. Exploring alternate electrolyzer designs, catalysts, and operational settings may also provide further chances to improve power efficiency.
IX. Conclusion
1. Higher electrolyte concentrations can enhance the efficiency of the electrolysis process.
2. Temperature enhances ion mobility, reduces activation energy, and improves mass transfer, leading to a more efficient electrolysis process.
3. Power efficiency of the electrolysis process varied significantly depending on the conditions and parameters applied.
4. Need more variable of experiment to compare and analysis.
5. Selecting the appropriate applied voltage and current density is crucial in determining the power efficiency of hydrogen electrolysis.
For Docs Mode, Click this link [2]
For Presentation Mode, Click this link [3]