Difference between revisions of "Theodore Binsar Putra Simamora"
(→Class Project) |
|||
| (23 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
| + | |||
| + | == About myself == | ||
| + | |||
[[File:Theodore Binsar Wiki.jpg]] | [[File:Theodore Binsar Wiki.jpg]] | ||
| − | + | ||
'''Name''' : Theodore Binsar Putra Simamora | '''Name''' : Theodore Binsar Putra Simamora | ||
| Line 16: | Line 19: | ||
The sugarcane company may supply sugarcane bagasse or molasses as a feedstock to the petroleum oil & gas company to produce biofuels, such as ethanol or biodiesel. This can be an example of a circular economy where waste from one industry is used as a resource in another industry. | The sugarcane company may supply sugarcane bagasse or molasses as a feedstock to the petroleum oil & gas company to produce biofuels, such as ethanol or biodiesel. This can be an example of a circular economy where waste from one industry is used as a resource in another industry. | ||
| − | The automotive factory may rely on petroleum oil & gas | + | The automotive factory may rely on petroleum oil & gas companies to supply the fuel for the vehicles it produces. The petroleum oil & gas company may also supply lubricants, coolants, and other chemicals that the automotive factory uses in its production processes. This can be an example of a traditional supply chain relationship where one company supplies goods to another company. |
The sugarcane company may supply ethanol, a biofuel derived from sugarcane, to the automotive factory. Ethanol can be blended with gasoline to make a fuel that is used in flexible fuel vehicles. This can be an example of a biofuels value chain where different companies along the value chain collaborate to produce and distribute a biofuel product. | The sugarcane company may supply ethanol, a biofuel derived from sugarcane, to the automotive factory. Ethanol can be blended with gasoline to make a fuel that is used in flexible fuel vehicles. This can be an example of a biofuels value chain where different companies along the value chain collaborate to produce and distribute a biofuel product. | ||
| Line 24: | Line 27: | ||
Overall, the relationship between these companies can be complex and multifaceted, and there may be various opportunities for collaboration or competition, depending on the specific circumstances. Understanding these relationships can be important for companies to identify potential risks and opportunities in their industry and make informed business decisions. | Overall, the relationship between these companies can be complex and multifaceted, and there may be various opportunities for collaboration or competition, depending on the specific circumstances. Understanding these relationships can be important for companies to identify potential risks and opportunities in their industry and make informed business decisions. | ||
| − | '''Theodore Binsar Putra Simamora''': I want you to act as an expert, explain the concept and reasons | + | '''Theodore Binsar Putra Simamora''': I want you to act as an expert, explain the concept and reasons for using ethanol/biofuel as an alternative |
'''Chatgpt''': Sure, I'd be happy to act as an expert and explain the concept and reasons for using ethanol/biofuel as an alternative. | '''Chatgpt''': Sure, I'd be happy to act as an expert and explain the concept and reasons for using ethanol/biofuel as an alternative. | ||
| Line 49: | Line 52: | ||
Additionally, the energy balance of ethanol production can be influenced by several factors, including the efficiency of the conversion process, the energy source used to power production facilities, and the transportation of the feedstock and finished product. | Additionally, the energy balance of ethanol production can be influenced by several factors, including the efficiency of the conversion process, the energy source used to power production facilities, and the transportation of the feedstock and finished product. | ||
| − | |||
| − | |||
| − | [[File: | + | == CCIT Visit == |
| + | Today, I and my classmates visited the CCIT workshop located not far from Universitas Indonesia. We learned about a mobile power plant, the Azolla plant, and further knowledge about pyrolisis. It was a wonderful visit and I thank Mr. DAI for the warm welcome. | ||
| + | |||
| + | [[File:visitCCIT.jpg|600px|thumb|center|visiting CCIT]] | ||
| + | |||
| + | [[File:bagasCCIT.jpg|400px|thumb|center|Bagas at CCIT]] | ||
| + | [[File:visitCCIT2.jpg|800px|thumb|center|on top of the world]] | ||
== Class Project == | == Class Project == | ||
| + | Electrolysis is the process of using an electric current to drive a chemical reaction. In the case of hydrogen production by electrolysis, water (H2O) is split into its component elements, hydrogen (H2) and oxygen (O2), using an electric current. | ||
| + | |||
| + | The theory behind hydrogen production by electrolysis is based on the principles of electrochemistry. When an electric current is passed through a solution, it causes the ions in the solution to move toward the electrodes. At the cathode (negative electrode), hydrogen ions (H+) gain electrons from the electrode and are reduced to form hydrogen gas (H2). At the anode (positive electrode), hydroxide ions (OH-) lose electrons to the electrode and are oxidized to form oxygen gas (O2) and water (H2O). | ||
| + | |||
| + | The overall reaction for hydrogen production by electrolysis is: | ||
| + | |||
| + | 2H2O(l) → 2H2(g) + O2(g) | ||
| + | |||
| + | This reaction requires an input of energy in the form of electricity to drive the process. The amount of energy required is related to the voltage applied to the system, the current flowing through the system, and the efficiency of the electrodes used. | ||
| + | |||
| + | The electrodes used in hydrogen production by electrolysis are typically made of a conductive material, such as platinum or graphite, that can withstand the harsh chemical environment of the electrolysis process. The electrodes are typically separated by a membrane or electrolyte solution that allows the flow of ions between the electrodes while preventing the mixing of the hydrogen and oxygen gases produced. | ||
| + | |||
| + | Hydrogen production by electrolysis is a promising method for producing hydrogen from renewable energy sources, such as solar or wind power, as it allows for the storage of excess energy in the form of hydrogen gas. However, the process is still relatively expensive compared to other methods of hydrogen production, such as steam methane reforming, and further research is needed to improve the efficiency and reduce the cost of the process. | ||
| + | |||
'''24/03/2023''' | '''24/03/2023''' | ||
| − | The link | + | |
| + | After a thorough discussion with the whole class, a joint experiment program led by Rasendriya in experimenting with hydrogen electrolysis is conducted. Preparation of the experiment includes finding a couple sheets of metal as the electrodes. I have chosen a 15 by 25 cm Aluminium 1100 plate as my plate. I decided to cut the aluminum metal plate by myself. The cutting process took place at Pusat Kegiatan Mahasiswa Universitas Indonesia. Here is attached the link for the youtube video containing my cutting process: https://youtube.com/shorts/Up2OZy_ux9Y?feature=share | ||
| + | |||
'''Hydrogen Electrolysis experiment (05/04/2023)''' | '''Hydrogen Electrolysis experiment (05/04/2023)''' | ||
| − | + | ||
| + | The experiment is conducted at Rasendriya's house. I, along with my classmates, arrived at 1 p.m. having already brought our metal plates. The experiment was successful and we acquired important data for the simulation. | ||
| + | |||
| + | [[File:rumahrasen.jpg|400px|thumb|center|Experiment at Rumah Rasen]] | ||
| + | |||
| + | [[File:platmetal.jpg|400px|thumb|center]] | ||
| + | |||
| + | There were 3 tests at 3 different voltages and currents. Each resulted in a different flow rate of Hydrogen. Here is the link to the video of the experiment and the result acquired, | ||
| + | |||
| + | Experiment video link: https://youtu.be/jqqNNHV48Pg | ||
| + | |||
| + | I personally picked the 80 ml/min data for the input of the Openmodelica simulation. There isn't anything special about this number or this segment of the experiment, I am simply picking it by random. | ||
| + | |||
| + | [[File:80ml-min.jpg|200px|thumb|center|Test result at 8 A and 19.3 V]] | ||
Voltage: 19.3 V | Voltage: 19.3 V | ||
Current: 8 A | Current: 8 A | ||
H2 flow: 80 ml/min. | H2 flow: 80 ml/min. | ||
| + | |||
| + | |||
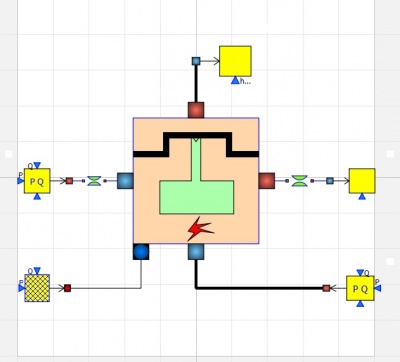
From this result, we can put the value for the input fuel parameter in OpenModelica to run the simulation of Internal Combustion Engine using hydrogen fuel. This is the model of the Internal Combustion Engine: | From this result, we can put the value for the input fuel parameter in OpenModelica to run the simulation of Internal Combustion Engine using hydrogen fuel. This is the model of the Internal Combustion Engine: | ||
| − | |||
| − | And this is the input parameter at the fuel source: | + | [[File:modelice.jpg|400px|thumb|center|Internal Combustion Engine Model]] |
| − | [[File:inputparameter.jpg]] | + | |
| + | And this is the input parameter at the fuel source on the fuelsourcePQ box at the lower left: | ||
| + | |||
| + | |||
| + | |||
| + | [[File:inputparameter.jpg|600px|thumb|center|Input parameter at fuelsourcePQ]] | ||
| + | |||
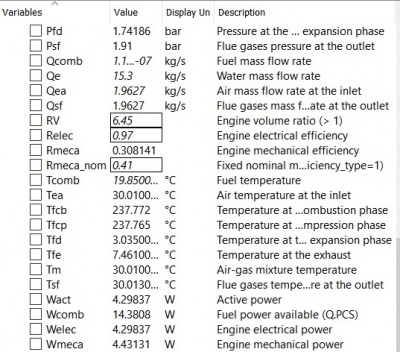
| + | The final results of mechanical engineering power produced are: | ||
| + | |||
| + | |||
| + | [[File:resultice.jpg|400px|thumb|center|Result]] | ||
| − | + | I would concern about the resulting mechanical engine power since it seems to be really low at about 4.4 Watts. I do not have an intuition as to how much energy can be produced from an 80mL/min hydrogen gas fuel source. However, the result is still in the realm of possibility, therefore I conclude this value is the final result. That is my part on this Internal Combustion Engine project, thanks to Rasen and other classmates that are really helpful throughout the duration of this experiment. Thank you. | |
| − | |||
Latest revision as of 23:36, 10 April 2023
About myself
Name : Theodore Binsar Putra Simamora
ID : 2006517871
Major : Mechanical Engineering
Chatgpt Conversations
Theodore: what is the relation between sugarcane company, automotive factory, and petroleum oil & gas company?
chatgpt :There may be several possible relationships between a sugarcane company, an automotive factory, and a petroleum oil & gas company depending on the context.
The sugarcane company may supply sugarcane bagasse or molasses as a feedstock to the petroleum oil & gas company to produce biofuels, such as ethanol or biodiesel. This can be an example of a circular economy where waste from one industry is used as a resource in another industry.
The automotive factory may rely on petroleum oil & gas companies to supply the fuel for the vehicles it produces. The petroleum oil & gas company may also supply lubricants, coolants, and other chemicals that the automotive factory uses in its production processes. This can be an example of a traditional supply chain relationship where one company supplies goods to another company.
The sugarcane company may supply ethanol, a biofuel derived from sugarcane, to the automotive factory. Ethanol can be blended with gasoline to make a fuel that is used in flexible fuel vehicles. This can be an example of a biofuels value chain where different companies along the value chain collaborate to produce and distribute a biofuel product.
The three companies may form a joint venture or partnership to produce biofuels or develop alternative fuels, such as hydrogen fuel cells, that could be used in both the automotive and industrial sectors. This can be an example of a strategic partnership where companies collaborate to achieve a common goal that they cannot achieve alone.
Overall, the relationship between these companies can be complex and multifaceted, and there may be various opportunities for collaboration or competition, depending on the specific circumstances. Understanding these relationships can be important for companies to identify potential risks and opportunities in their industry and make informed business decisions.
Theodore Binsar Putra Simamora: I want you to act as an expert, explain the concept and reasons for using ethanol/biofuel as an alternative
Chatgpt: Sure, I'd be happy to act as an expert and explain the concept and reasons for using ethanol/biofuel as an alternative.
Ethanol is a type of biofuel that is made from plant-based sources such as corn, sugarcane, or switchgrass. It is a renewable fuel that can be used as an alternative to gasoline in vehicles. The use of ethanol as a fuel has been gaining popularity in recent years due to several reasons.
One of the main reasons for using ethanol as an alternative is that it is a renewable source of energy. Unlike gasoline, which is a finite resource, ethanol can be produced from a variety of crops that can be grown each year. This makes ethanol a more sustainable and environmentally friendly option for fuel.
Another reason for using ethanol is that it produces fewer greenhouse gas emissions than gasoline. When ethanol is burned as fuel, it releases carbon dioxide, but the plants used to produce ethanol absorb carbon dioxide from the atmosphere during their growth. This means that the net carbon emissions from ethanol are lower than those from gasoline.
In addition to its environmental benefits, ethanol also has economic benefits. Ethanol production can create jobs in rural areas where crops are grown and processed. It can also reduce dependence on foreign oil and help to stabilize fuel prices.
There are some challenges associated with the use of ethanol as a fuel. One of the main challenges is that it has a lower energy density than gasoline, meaning that it takes more ethanol to travel the same distance as gasoline. This can result in lower fuel efficiency and higher fuel costs for consumers. There are also concerns about the impact of ethanol production on food prices and the availability of food crops.
Overall, the concept of using ethanol as an alternative fuel is based on its renewable nature, environmental benefits, and economic advantages. While there are challenges to its use, the potential benefits make it an attractive option for reducing dependence on fossil fuels and promoting sustainability.
Theodore Binsar Putra Simamora: are the use of ethanol energy positive or negative? Chatgpt: The use of ethanol as a fuel can be either energy positive or negative, depending on the specific production method and energy inputs used.
On one hand, ethanol produced from some feedstocks such as sugarcane or switchgrass, can have a positive energy balance, meaning that the amount of energy produced from the ethanol is greater than the energy required to produce it. This is because these feedstocks require less energy to grow and process into ethanol.
On the other hand, ethanol produced from corn, which is the most commonly used feedstock in the United States, has a lower energy balance and can be energy negative. This is because corn requires significant amounts of energy to grow and process into ethanol, which can offset some of the energy benefits of using the fuel.
Additionally, the energy balance of ethanol production can be influenced by several factors, including the efficiency of the conversion process, the energy source used to power production facilities, and the transportation of the feedstock and finished product.
CCIT Visit
Today, I and my classmates visited the CCIT workshop located not far from Universitas Indonesia. We learned about a mobile power plant, the Azolla plant, and further knowledge about pyrolisis. It was a wonderful visit and I thank Mr. DAI for the warm welcome.
Class Project
Electrolysis is the process of using an electric current to drive a chemical reaction. In the case of hydrogen production by electrolysis, water (H2O) is split into its component elements, hydrogen (H2) and oxygen (O2), using an electric current.
The theory behind hydrogen production by electrolysis is based on the principles of electrochemistry. When an electric current is passed through a solution, it causes the ions in the solution to move toward the electrodes. At the cathode (negative electrode), hydrogen ions (H+) gain electrons from the electrode and are reduced to form hydrogen gas (H2). At the anode (positive electrode), hydroxide ions (OH-) lose electrons to the electrode and are oxidized to form oxygen gas (O2) and water (H2O).
The overall reaction for hydrogen production by electrolysis is:
2H2O(l) → 2H2(g) + O2(g)
This reaction requires an input of energy in the form of electricity to drive the process. The amount of energy required is related to the voltage applied to the system, the current flowing through the system, and the efficiency of the electrodes used.
The electrodes used in hydrogen production by electrolysis are typically made of a conductive material, such as platinum or graphite, that can withstand the harsh chemical environment of the electrolysis process. The electrodes are typically separated by a membrane or electrolyte solution that allows the flow of ions between the electrodes while preventing the mixing of the hydrogen and oxygen gases produced.
Hydrogen production by electrolysis is a promising method for producing hydrogen from renewable energy sources, such as solar or wind power, as it allows for the storage of excess energy in the form of hydrogen gas. However, the process is still relatively expensive compared to other methods of hydrogen production, such as steam methane reforming, and further research is needed to improve the efficiency and reduce the cost of the process.
24/03/2023
After a thorough discussion with the whole class, a joint experiment program led by Rasendriya in experimenting with hydrogen electrolysis is conducted. Preparation of the experiment includes finding a couple sheets of metal as the electrodes. I have chosen a 15 by 25 cm Aluminium 1100 plate as my plate. I decided to cut the aluminum metal plate by myself. The cutting process took place at Pusat Kegiatan Mahasiswa Universitas Indonesia. Here is attached the link for the youtube video containing my cutting process: https://youtube.com/shorts/Up2OZy_ux9Y?feature=share
Hydrogen Electrolysis experiment (05/04/2023)
The experiment is conducted at Rasendriya's house. I, along with my classmates, arrived at 1 p.m. having already brought our metal plates. The experiment was successful and we acquired important data for the simulation.
There were 3 tests at 3 different voltages and currents. Each resulted in a different flow rate of Hydrogen. Here is the link to the video of the experiment and the result acquired,
Experiment video link: https://youtu.be/jqqNNHV48Pg
I personally picked the 80 ml/min data for the input of the Openmodelica simulation. There isn't anything special about this number or this segment of the experiment, I am simply picking it by random.
Voltage: 19.3 V Current: 8 A H2 flow: 80 ml/min.
From this result, we can put the value for the input fuel parameter in OpenModelica to run the simulation of Internal Combustion Engine using hydrogen fuel. This is the model of the Internal Combustion Engine:
And this is the input parameter at the fuel source on the fuelsourcePQ box at the lower left:
The final results of mechanical engineering power produced are:
I would concern about the resulting mechanical engine power since it seems to be really low at about 4.4 Watts. I do not have an intuition as to how much energy can be produced from an 80mL/min hydrogen gas fuel source. However, the result is still in the realm of possibility, therefore I conclude this value is the final result. That is my part on this Internal Combustion Engine project, thanks to Rasen and other classmates that are really helpful throughout the duration of this experiment. Thank you.