Difference between revisions of "Regina Calysta Natalie"
(→WORKSHOP VISIT) |
(→FINAL EXAM AND PROJECT FOR HYDROGEN) |
||
| (32 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | |||
== INTRODUCTION == | == INTRODUCTION == | ||
| Line 31: | Line 32: | ||
This topic is rather interesting as it is a vast topic which can be discussed endlessly regarding many sections. But as an engineer, creating better technology will help achieve sustainable energy. Still, it will only work if these other sections want the same thing. | This topic is rather interesting as it is a vast topic which can be discussed endlessly regarding many sections. But as an engineer, creating better technology will help achieve sustainable energy. Still, it will only work if these other sections want the same thing. | ||
| − | == Fuel for Thought? == | + | == Fuel for Thought? Go HYDRO! == |
'''23/02/2023''' | '''23/02/2023''' | ||
| Line 53: | Line 54: | ||
---- | ---- | ||
| + | '''14/03/2023''' | ||
| + | I got to in front of the class to explain regarding my personal project progress. From there I got to have new perspectives from Prof. Adi regarding my output. | ||
| + | |||
| + | ---- | ||
'''VIDEO PRESENTATION''' | '''VIDEO PRESENTATION''' | ||
https://youtu.be/dcaxDk1pdX8 | https://youtu.be/dcaxDk1pdX8 | ||
| + | |||
| + | ---- | ||
| + | '''17/03/2023''' | ||
| + | |||
| + | '''After going through several discussion, the personal project has been shifted into one my classmate's project to vary the parameters input for Hydrofuel that was achieved through the process of hydrolysis'''. On Friday, I got to do my first quiz in Pak DAI's class with simulating the IC Engine itself. There was a lot of mistakes that I made especially in the fuel source input. | ||
| + | |||
| + | The results that were achieved were also need to be fixed: | ||
| + | [[File:QuizRegina1.jpg|500px|thumb|center|Quiz Regina Page 1]] | ||
| + | |||
| + | [[File:QuizRegina2.jpg|500px|thumb|center|Quiz Regina Page 2]] | ||
| + | |||
| + | ---- | ||
| + | '''21/03/2023''' | ||
| + | |||
| + | For Tuesday, I organized a chatting with Rasendriya especially for the whole class regarding the hydro fuel project. We got to assign the variations between the metal plates for the hydrolysis process. For me personally, I picked 0.8 mm in thickness with the dimension of 20 x 25 cm. The list for the whole class can be seen in this link: [https://docs.google.com/spreadsheets/d/1-XlqcXZdlD3rF8RzLliO9MfI9Ozu0__5IXlepA0aIIE/edit?usp=sharing List Plate Variations] | ||
| + | |||
| + | ---- | ||
| + | '''24/03/2023''' | ||
| + | |||
| + | For today, I got to perfect my modeling in IC Engine, especially for the hydro fuel project. The presentation can be seen in this link below: | ||
| + | |||
| + | [https://youtu.be/MmsRy7BgURs Regina's Personal Project Progress] | ||
| + | |||
| + | [[File:PPTScreensot.png|500px|thumb|center|Go HYDRO PPT]] | ||
| + | |||
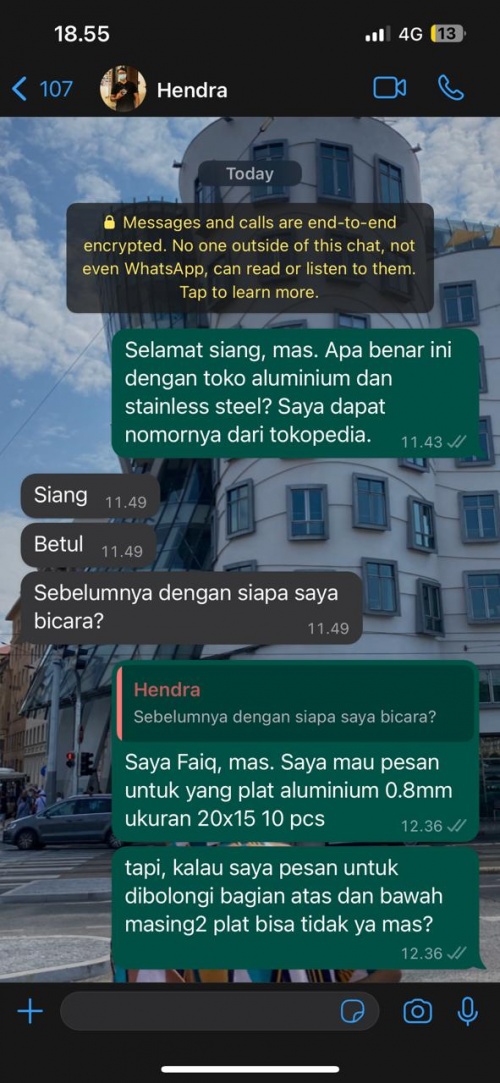
| + | For the plate variations, I planned with the whole class to do it on Sunday. For now, I decided to order the same plate thickness as Faiq. Faiq and I already asked to a vendor for the Aluminum 1100 on Tokopedia, but the seller hasn't replied us back again. We planned to search it in offline stores over the weekend and perfecting our plate models by time that can be used next Tuesday. | ||
| + | |||
| + | [[File:Chat.jpg|500px|thumb|center|Faiq's Chat with Our Vendor]] | ||
| + | |||
| + | [[File:GroupChat.png|500px|thumb|center|Mesin KKI 19 Group Work Appointment]] | ||
| + | ---- | ||
| + | |||
| + | '''MIDTERM PROGRESS AND PRESENTATION ELECTROLYSIS''' | ||
| + | |||
| + | The link to my report will be shown below | ||
| + | [https://docs.google.com/document/d/13M7KODv1l8SAXWM98c7nOBTYyzhZex9AYDf_wu7xQNc/edit?usp=sharing Regina Calysta Natalie's Electrolysis Report] | ||
| + | |||
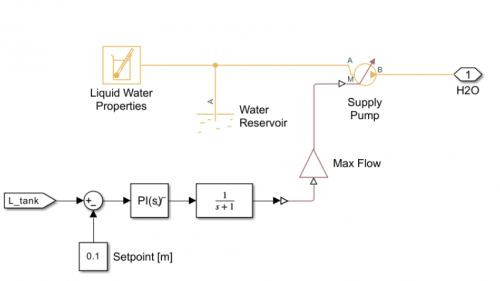
| + | For the modelling, I use MATLAB and OpenModelica. | ||
| + | |||
| + | '''MATLAB''' | ||
| + | [[File:matlab1.png|500px|thumb|center|Electrolysis Model]] | ||
| + | |||
| + | 1. Import “ssc_electrolyzer” code in the MATLAB. | ||
| + | |||
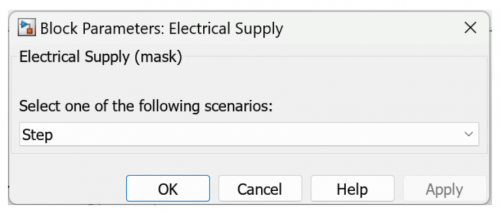
| + | 2. Edit the Electrical Supply into Step with the specifications of initial voltage 6 - 10 Volt and step time of 3600. | ||
| + | [[File:ssc.png|500px|thumb|center|Electrical Supply Parameters]] | ||
| + | 3. Edit the water supply’s setpoint | ||
| + | [[File:waters.png|500px|thumb|center|Electrical Supply Parameters]] | ||
| + | 4. Edit the Membrane Electrode details | ||
| + | [[File:electrode.png|500px|thumb|center|Membrane Electrode Assembly Details]] | ||
| + | 5. Insert the stop time to 10s | ||
| + | |||
| + | 6. Run the simulation. | ||
| + | |||
| + | '''OpenModelica''' | ||
| + | |||
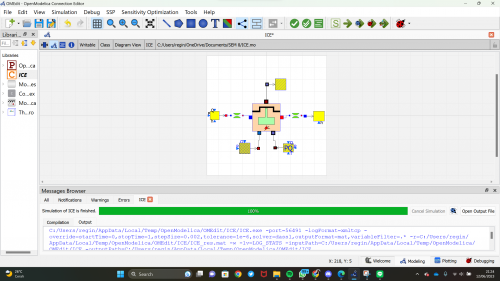
| + | 1. Open the ThermoSysPro Library and make the model | ||
| + | [[File:OMIC.png|500px|thumb|center|OpenModelica IC Engine Design]] | ||
| + | |||
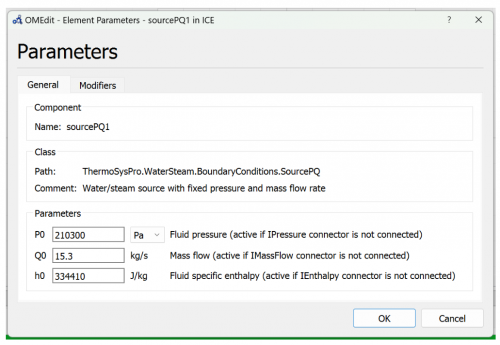
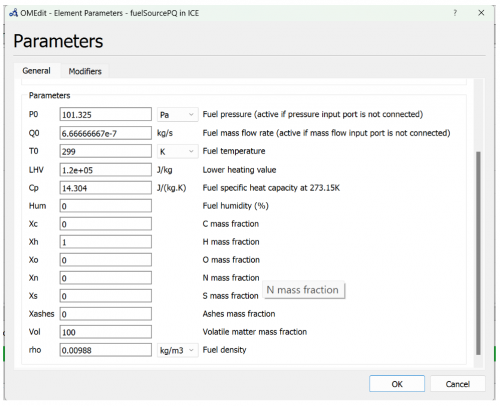
| + | 2. Enter the parameter based on these figures below | ||
| + | [[File:OMIC1.png|500px|thumb|center|IC Engine Parameter]] | ||
| + | |||
| + | [[File:OMIC2.png|500px|thumb|center|Cooling Source Parameter]] | ||
| + | |||
| + | [[File:OMIC3.png|500px|thumb|center|Fuel Source Parameter]] | ||
| + | |||
| + | |||
| + | The end result can be seen in the figure below | ||
| + | [[File:OMIC4.png|500px|thumb|center|End Result]] | ||
| + | |||
| + | ---- | ||
== Open Modelica IC Engine Tutorial / 24 February 2023 == | == Open Modelica IC Engine Tutorial / 24 February 2023 == | ||
| Line 115: | Line 191: | ||
| − | == | + | == Pyrolysis Introduction / 3 March 2023 == |
| − | + | Pyrolysis is a thermo-chemical conversion method that involves the decomposition of organic or inorganic materials into solid, liquid, and gaseous products. Pyrolysis is a complex process that involves multiple chemical reactions, and the products derived from the process are highly dependent on the type of feedstock and operating parameters used. Pyrolysis can be achieved through different routes, including gasification, liquefaction, and combustion. The main difference between these routes is the type of products they produce and the process used to generate them. | |
| − | |||
| − | - | ||
| − | + | One of the advantages of pyrolysis is that it can create different products depending on the operating parameters used. For example, in pyrolysis, little to no oxygen is used in the combustion process, which creates char, gas, and oil. However, other conversion methods, such as gasification, combustion, and liquefaction, can create different products such as gas and liquid. | |
| − | + | The type of feedstock used in pyrolysis also plays a significant role in the process and output. Various feedstocks can be used, including municipal solid waste, plastic and polymer, lignocellulosic materials, sewage sludge, and paper waste. Each of these feedstocks can result in different output products, depending on the operating parameters used. | |
| − | - | + | The pyrolysis process requires high temperatures, and different heating sources can be used, such as furnaces, steam, heating tapes, and microwaves. The choice of heating source can affect the heating rate and heat transfer mechanism, and the reactor needs to be designed accordingly to maximize efficiency. Reactors used in pyrolysis include fixed-bed, moving bed, and microwave-induced pyrolysis. |
| − | + | In conclusion, pyrolysis is a useful conversion method for biomass, as it can create different products depending on the operating parameters used. Pyrolysis is a complex process that involves multiple chemical reactions, and the type of feedstock and operating parameters used can significantly affect the output. The choice of heating source and reactor design also plays a crucial role in maximizing efficiency. | |
| − | + | In class, we also talked about Prof. Adi's project: Desalination, Biomass, Pyrolysis, Drier which will be discussed next meeting. | |
| − | + | == Desalination and Pyrolysis = Working in Synergy / 7 March 2023 == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
- Desalination = Indonesian geography --> Drinkable water is decreasing by the hour. | - Desalination = Indonesian geography --> Drinkable water is decreasing by the hour. | ||
| Line 172: | Line 223: | ||
- Drier | - Drier | ||
| + | |||
| + | [[File:Prof. Adi Diagram.jpg|500px|thumb|center|Prof. Adi's Diagram]] | ||
| + | |||
| + | Desalination is a process that can provide clean drinking water by removing salt and other impurities from seawater. However, the process requires a lot of energy, and the use of fossil fuels to power desalination plants can contribute to waste and pollution. One solution to this problem is the use of biomass as a sustainable source of energy. Biomass, such as organic waste, can be converted into energy through pyrolysis, where the waste is heated without oxygen to produce biochar or other products. Using biomass as a fuel source for desalination plants, we can reduce waste and pollution while also providing a sustainable energy source. This approach not only helps to provide clean drinking water but also supports the circular economy by reducing waste and contributing to a more sustainable future. | ||
== WORKSHOP VISIT == | == WORKSHOP VISIT == | ||
| Line 199: | Line 254: | ||
[[File:Regina CCIT.jpg|500px|thumb|center|Regina Selfie]] | [[File:Regina CCIT.jpg|500px|thumb|center|Regina Selfie]] | ||
| + | |||
| + | |||
| + | == FINAL EXAM AND PROJECT FOR HYDROGEN == | ||
| + | |||
| + | {| Class ='wikitable' | ||
| + | | "Seeing and Feeling is BELIEVING" - DAI | ||
| + | |} | ||
| + | |||
| + | |||
| + | Midterm Progress can be seen in this section: | ||
| + | |||
| + | [https://air.eng.ui.ac.id/index.php?title=Regina_Calysta_Natalie#Fuel_for_Thought.3F_Go_HYDRO.21 REGINA'S MIDTERM] | ||
| + | |||
| + | |||
| + | '''Abstract:''' | ||
| + | |||
| + | This study focused on the experimentation and analysis of hydrogen obtained through plate variation. The hydrogen was burned continuously using a one-way valve and small nozzle, resulting in a steady flame capable of boiling water. By measuring the temperature increase of water placed on a spoon, the energy gained from the experiment was determined. Additionally, the mechanical and electrical power generated were calculated. A comparison between the experimental and ideal conditions revealed slight discrepancies, likely due to the safety precautions of conducting the experiments in an open environment. These findings highlight the potential for energy production and emphasize the need for further optimization and refinement of experimental conditions to achieve more precise results. | ||
| + | |||
| + | [[File:hydro 7 june.jpg|200px|thumb|center|Experimentation Setup]] | ||
| + | |||
| + | '''For the full report, everything is written in here including the results and analyses''': | ||
| + | [https://docs.google.com/document/d/13M7KODv1l8SAXWM98c7nOBTYyzhZex9AYDf_wu7xQNc/edit?usp=sharing Regina Calysta Natalie's Electrolysis Report] | ||
| + | |||
| + | On June 7, 2023, the study progressed by conducting experiments on the hydrogen obtained through plate variation, with a flow rate of 6.66666667e-7 kg/s. The research involved burning the hydrogen, which was safely distributed via a one-way valve and small nozzle. The continuous supply of hydrogen created a consistent flame capable of boiling water. The boiled water, placed on a spoon weighing 0.014 kg, experienced a temperature increase of approximately 27 degrees Celsius, rising from 28 degrees to 55 degrees. Upon calculations, it was determined that the experiment yielded a total of 1587.6 Joules of energy and 113,400 J/kg as a specific result. | ||
| + | |||
| + | [[File:OM final.png|300px|thumb|center|OM Final]] | ||
| + | |||
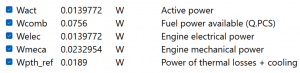
| + | The subsequent step involves inputting the actual values into the Open Modelica setting, enabling us to obtain the genuine results. Through modeling, it becomes apparent that the achievable mechanical power amounts to 0.0023 Watt, while the electric power reaches 0.0139 Watt. However, there is a slight loss when comparing these results to the simulation outcomes in Open Modelica. In an ideal scenario, the mechanical power could reach 0.0025 Watt, and the electrical power would be 0.015 Watt. Additionally, it is worth noting that the experiment conducted produced approximately 1600 Joules of energy, while the ideal conditions, as per the Matlab simulation, could generate approximately 9000 Joules or 0.0025 kWh. This loss can be attributed to conducting the experimentation in an open environment to ensure our own safety. | ||
| + | |||
| + | [[File:OM final results.png|300px|thumb|center|OM Final Results]] | ||
| + | |||
| + | In conclusion, the experiment using an Aluminium 1100 cathode with a dimension of 5 x 10 mm and a total of 8 pieces, and varying the current between 6A, 8A, and 10A, with NaOH as the electrolyte, has proven the practicality and potential of electrolysis for generating hydrogen gas from water. There are 3 factors that play a part in the efficiency of how much hydrogen is produced: current flowing, material of the plates, dimension of the plates. More investigation and advancement in this area, including examining the impact of various electrode materials, optimising the current and voltage applied, and exploring the effects of different electrolyte concentrations, may lead to innovative and new uses of this technology, which can assist in addressing the challenges related to energy storage and sustainable energy production. | ||
| + | |||
| + | Furthermore, the experimentation process involved studying the hydrogen gained through plate variation, resulting in the continuous burning of hydrogen that produced a consistent flame capable of boiling water. The analysis of the boiled water revealed a significant increase in temperature. Further calculations showed the energy gained from the experiment, as well as the mechanical and electrical power produced. While there were slight discrepancies between the experimental and ideal conditions, these variations were expected due to the open environment in which the experiments were conducted for safety purposes. Overall, the study provided valuable insights into the potential energy production and demonstrated the importance of further refining and optimising the experimental conditions for more accurate results. | ||
| + | |||
| + | '''FINAL PROJECT SLIDES''': | ||
| + | |||
| + | [https://www.canva.com/design/DAFfxLesyAI/-Yr64oGbVYSzdVjVGdptGA/view?utm_content=DAFfxLesyAI&utm_campaign=designshare&utm_medium=link&utm_source=publishsharelink REGINA'S FINAL YEAR PROJECT SLIDES] | ||
| + | |||
| + | |||
| + | '''VIDEO PRESENTATION''': | ||
| + | [https://www.youtube.com/watch?v=0VoR23Nh28g FINAL PROJECT PRESENTATION] | ||
Latest revision as of 23:37, 12 June 2023
Contents
- 1 INTRODUCTION
- 2 Correlations between Industries and Energy / 21 February 2023
- 3 Fuel for Thought? Go HYDRO!
- 4 Open Modelica IC Engine Tutorial / 24 February 2023
- 5 Vibrations and Frequencies / 28 February 2023
- 6 Pyrolysis Introduction / 3 March 2023
- 7 Desalination and Pyrolysis = Working in Synergy / 7 March 2023
- 8 WORKSHOP VISIT
- 9 FINAL EXAM AND PROJECT FOR HYDROGEN
INTRODUCTION
Hello, my name is Regina. I am a student in ECS 2. This will be my page regarding ECS2.
FULLNAME: Regina Calysta Natalie
STUDENT NUMBER: 1906315834
Correlations between Industries and Energy / 21 February 2023
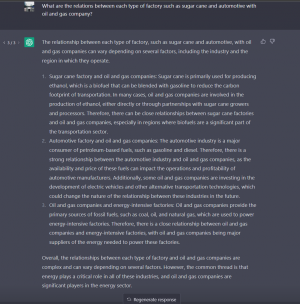
After chatting with chatGPT regarding relations between each type of factory with oil and gas company, as shown in the picture below, the chatGPT has replied with an exciting answer.
In conclusion, the industries shown have various relationships with oil and gas companies. There can be a close relationships between factories such as sugar factory and oil and gas company as they both produce ethanol therefore biofuels will be a significant part of the transportation sector. Additionally, a strong correlation exists as the automotive industry mainly consumes petroleum-based fuels. In short terms, oil and gas companies provide the primary sources of fossil fuels to power up these factories and therefore that it plays a critical role.
After knowing such critical role, then where does oil and gas companies play a part in sustainability energy transition? I asked chatGPT regarding this question, which was the AI's answer.
In conclusion, oil and gas companies have a complex role in the sustainability movement, as they are major contributors to climate change and other environmental issues. Despite this, some companies promote sustainability by investing in renewable energy sources, developing low-carbon technologies, setting emissions reduction targets, and participating in sustainability initiatives. While some argue that more needs to be done to address the environmental impact of these companies, their participation in sustainability initiatives and investment in renewable energy and low-carbon technologies suggest they are beginning to recognize the importance of sustainability in the energy sector.
There is an article worth reading regarding this matter from IEA (International Energy Agency). The Oil and Gas Industry in Energy Transitions
It highlights the challenges and opportunities the oil and gas industry faces in transitioning to a low-carbon energy system. The report also emphasizes the importance of collaboration between governments, the industry, and other stakeholders to address climate change and achieve sustainable energy goals. Several key factors outlines the report including the growth of renewable energy and the need to reduce greenhouse gas emissions. There are also several strategies that the industries can adapt to the changing energy landscape to support sustainability. Overall, this article emphasizes the need for oil and gas industry to take a proactive steps to address the challenges of energy transitions and work towards a sustainable energy usage.
This topic is rather interesting as it is a vast topic which can be discussed endlessly regarding many sections. But as an engineer, creating better technology will help achieve sustainable energy. Still, it will only work if these other sections want the same thing.
Fuel for Thought? Go HYDRO!
23/02/2023
My initial thought is to look at my Honda Jazz and research its fuel consumption based on my usual route, Tangerang to UI. But I am also curious regarding the literal difference from Honda Jazz that uses VTEC and those who do not use VTEC. So I will have a look on that as well.
27/02/2023
Ever since classes have started going on offline, my only main way of transport is by going on in car or going on a train. But since most of my classes started early, I decided to go on by my car. Only after regularly using my car from Monday to Friday, I then realized how much in terms of price that I need to fill up my whole tank. My car is 2016 All New Honda Jazz with automatic transmission which can be seen down below.
This Honda Jazz generally has a capacity of 40L of fuel for the tank itself. The distance from my house to my campus is considered pretty far around 50 km for one ride and another 50 km which totals into around 100~110 km of distance. Back in late 2022, I noticed that my fuel usage is considered pretty economic since the ratio that I typically get is around 1 L for around 16~17 km. But lately, there has been changes in the fuel consumption.
My personal project for this course is to investigate what could be the trigger for this change from literature review and also looking at the engine performance itself based on the last shop visit. Other than that, I will also take a look on the analysis between whether or not VTEC engine also plays a part in the fuel consumption itself.
For the calculation, I will try to look for fuel consumption modeling using Modelica based on this article: Package PowerTrain: A Modelica library for modeling and simulation of vehicle power trains
14/03/2023
I got to in front of the class to explain regarding my personal project progress. From there I got to have new perspectives from Prof. Adi regarding my output.
VIDEO PRESENTATION
17/03/2023
After going through several discussion, the personal project has been shifted into one my classmate's project to vary the parameters input for Hydrofuel that was achieved through the process of hydrolysis. On Friday, I got to do my first quiz in Pak DAI's class with simulating the IC Engine itself. There was a lot of mistakes that I made especially in the fuel source input.
The results that were achieved were also need to be fixed:
21/03/2023
For Tuesday, I organized a chatting with Rasendriya especially for the whole class regarding the hydro fuel project. We got to assign the variations between the metal plates for the hydrolysis process. For me personally, I picked 0.8 mm in thickness with the dimension of 20 x 25 cm. The list for the whole class can be seen in this link: List Plate Variations
24/03/2023
For today, I got to perfect my modeling in IC Engine, especially for the hydro fuel project. The presentation can be seen in this link below:
Regina's Personal Project Progress
For the plate variations, I planned with the whole class to do it on Sunday. For now, I decided to order the same plate thickness as Faiq. Faiq and I already asked to a vendor for the Aluminum 1100 on Tokopedia, but the seller hasn't replied us back again. We planned to search it in offline stores over the weekend and perfecting our plate models by time that can be used next Tuesday.
MIDTERM PROGRESS AND PRESENTATION ELECTROLYSIS
The link to my report will be shown below Regina Calysta Natalie's Electrolysis Report
For the modelling, I use MATLAB and OpenModelica.
MATLAB
1. Import “ssc_electrolyzer” code in the MATLAB.
2. Edit the Electrical Supply into Step with the specifications of initial voltage 6 - 10 Volt and step time of 3600.
3. Edit the water supply’s setpoint
4. Edit the Membrane Electrode details
5. Insert the stop time to 10s
6. Run the simulation.
OpenModelica
1. Open the ThermoSysPro Library and make the model
2. Enter the parameter based on these figures below
The end result can be seen in the figure below
Open Modelica IC Engine Tutorial / 24 February 2023
I haven't installed OpenModelica so for today, I only took notes on what we did today.
Today, we received a tutorial regarding OpenModelica for IC Engine with Bang Tanwir. IC Engine is an internal combustion engine with fuel and air to be combusted with power and fuel gasses output. IC Engine also needs cooling with refrigerant. In OMEdit, there is a library that consists of IC Engine, therefore we can import the library. Here are the steps for IC Engine modelling in Open Modelica:
1. Open OM. Import the Library. The library name is ThermoSysPro 3.1 or ThermoSysPro 3.2 -> package.mo
2. Create new modelica class "c_engine".
3. Drag the Engine to the workplace.
4. For the left and right, we add the cooling: can be air or water
5. FuelSourcePQ (inside Boundary Conditions) is added to the source inlet or gas connector.
6. SourcePQ is connected to the air inlet for water cooling.
7. SourcePQ is connected to the cooling inlet.
8. Seek is connected to the right side of the cooling (outlet).
9. Add Singular Pressure Loss (shapes like an hourglass) in the middle between Source PQ and cooling inlet and in the middle of the cooling outlet and seek.
10. Input the variables based on the reference. A reference that can be looked at: Study Case IC Engine in CCIT
Vibrations and Frequencies / 28 February 2023
During today's lecture, Professor Adi covered several topics related to energy conversion, including IC engines, pyrolysis, and electric vehicles (EVs). In particular, he discussed the energy conversion process that occurs when using fuels like LPG or gasoline in engines. The conversion of chemical energy in these fuels into mechanical energy happens due to the vibration of atoms and molecules, which creates heat and pressure that is used to power the engine. Professor Adi used the example of hitting a table to create sound as an analogy to illustrate this concept.
Pyrolysis, on the other hand, is a thermal decomposition process that does not involve combustion. Instead, organic materials such as biomass, plastics, or rubber are heated without oxygen, breaking them into smaller molecules. This process can produce various valuable products, including biofuels, activated carbon, and other chemicals. Finally, Professor Adi discussed EVs as a more sustainable alternative to combustion engines since they run on electricity.
Combustion is a chemical reaction between a fuel and an oxidizer that produces heat and often light. The most common type of combustion is the reaction between a hydrocarbon fuel (such as methane, propane, or gasoline). Combustion is a type of energy conversion process because it involves converting chemical energy stored in the fuel into thermal energy. This process is widely used in many industries, including transportation, power generation, and manufacturing. However, combustion can also produce harmful byproducts, such as carbon monoxide and nitrogen oxides, which can have adverse environmental and health effects. As a result, there is a growing interest in developing alternative energy conversions processes, such as fuel cells or renewable energy sources, that can help reduce combustion's negative impact on the environment.
| "If you want to understand the nature, think of energy, vibration, and frequency." - Nikola Tesla |
From the quotation on the above, we have to think back to the basic which are vibration and frequency. The important question is "What are the conclusions from the previous topic?"
| "In conclusion, it is interesting how all topics are connected if we think back about the basics such as energy and vibration." |
In today's lecture, Prof. Adi also mentioned regarding plastics' composition. Plastics are synthetic polymers made from a variety of materials such as crude oil, natural gas, coal, cellulose, and other petrochemicals. The process of making plastics involves chemically bonding together molecules to form long chains known as polymers. Depending on the type of plastic, various additives such as stabilizers, pigments, and plasticizers can be added to the mixture to enhance their properties. The chemical composition of plastics varies depending on the type of plastic. Most plastics are made up of long chains of hydrocarbon molecules, which consist of carbon and hydrogen atoms. These hydrocarbon chains can be linear, branched, or cross-linked, and their physical properties depend on the length and structure of the chains. Other chemical elements such as oxygen, nitrogen, chlorine, or sulfur can also be present in some types of plastics, adding further complexity to their composition.
It is essential for people to understand the purpose of plastics especially trash and how they can contribute to the environment. With proper knowledge and education, individuals can take steps to reduce their plastic use, recycle and dispose of plastic waste properly, and support initiatives that promote sustainable plastics. The purpose can be gained not only from higher education but also from various sources such as media, public campaigns, or personal experiences. For example, Nikola Tesla, a renowned inventor and engineer, gained his purpose from his fascination with electricity and a desire to make the world a better place.
One of the pressing environmental issues is the increasing amount of waste, including plastic waste, generated by human activities. Developed countries have implemented various ways of managing waste, such as recycling, incineration, or landfilling, to minimize the negative impact on the environment. However, developing countries, such as Indonesia, are still struggling to find effective waste management strategies. It is crucial to create awareness and develop innovative solutions to tackle this issue.
In the field of technology, the evolution of mobile communication technology has led to the development of various generations of mobile networks, from 2G to 5G. The primary difference between these networks is the frequency range and bandwidth they use, which affects the speed and quality of data transmission. As technology advances, the demand for higher data speeds and more reliable networks continues to grow, leading to the development of newer generations of mobile networks.
Pyrolysis Introduction / 3 March 2023
Pyrolysis is a thermo-chemical conversion method that involves the decomposition of organic or inorganic materials into solid, liquid, and gaseous products. Pyrolysis is a complex process that involves multiple chemical reactions, and the products derived from the process are highly dependent on the type of feedstock and operating parameters used. Pyrolysis can be achieved through different routes, including gasification, liquefaction, and combustion. The main difference between these routes is the type of products they produce and the process used to generate them.
One of the advantages of pyrolysis is that it can create different products depending on the operating parameters used. For example, in pyrolysis, little to no oxygen is used in the combustion process, which creates char, gas, and oil. However, other conversion methods, such as gasification, combustion, and liquefaction, can create different products such as gas and liquid.
The type of feedstock used in pyrolysis also plays a significant role in the process and output. Various feedstocks can be used, including municipal solid waste, plastic and polymer, lignocellulosic materials, sewage sludge, and paper waste. Each of these feedstocks can result in different output products, depending on the operating parameters used.
The pyrolysis process requires high temperatures, and different heating sources can be used, such as furnaces, steam, heating tapes, and microwaves. The choice of heating source can affect the heating rate and heat transfer mechanism, and the reactor needs to be designed accordingly to maximize efficiency. Reactors used in pyrolysis include fixed-bed, moving bed, and microwave-induced pyrolysis.
In conclusion, pyrolysis is a useful conversion method for biomass, as it can create different products depending on the operating parameters used. Pyrolysis is a complex process that involves multiple chemical reactions, and the type of feedstock and operating parameters used can significantly affect the output. The choice of heating source and reactor design also plays a crucial role in maximizing efficiency.
In class, we also talked about Prof. Adi's project: Desalination, Biomass, Pyrolysis, Drier which will be discussed next meeting.
Desalination and Pyrolysis = Working in Synergy / 7 March 2023
- Desalination = Indonesian geography --> Drinkable water is decreasing by the hour.
- How to slowly decrease the waste that are being thrown into the sea. In a certain area, the waste must be cleaned first before doing the desalination process especially if the sea water is going to be converted into drinkable water. The waste needs to be picked up by kapal peluk sampah laut.
- Not a lot of people care about the important of drinkable water and the importance of desalination.
- In desalination, there's a mechanism that can filter the sea water.
- Interesting video regarding the discussion whether desalination process could save the world's water crisis: Can sea water desalination save the world?
- Desalination method = filtration, graphene ; Water desalination through capacitive deionization using graphene-based electrodes
- Biomass = organic waste, Charcoal, biochar
- Pyrolysis
- Drier
Desalination is a process that can provide clean drinking water by removing salt and other impurities from seawater. However, the process requires a lot of energy, and the use of fossil fuels to power desalination plants can contribute to waste and pollution. One solution to this problem is the use of biomass as a sustainable source of energy. Biomass, such as organic waste, can be converted into energy through pyrolysis, where the waste is heated without oxygen to produce biochar or other products. Using biomass as a fuel source for desalination plants, we can reduce waste and pollution while also providing a sustainable energy source. This approach not only helps to provide clean drinking water but also supports the circular economy by reducing waste and contributing to a more sustainable future.
WORKSHOP VISIT
On Friday, March 10 2023, we got to visit Pak DAI's workshop CCIT based in Kukusan. In there we got to have an introduction to IC Engine and Pyrolysis in MRPP (Mobile Refinery and Power Plant). Other that that we got to know Pak DAI's workshop in CCIT. Bang Tanwir and Bu Illa got to introduce us to MRPP.
MRPP has 3 process = Genset, Pyrolysis, and FCC (Fluid Catalytic Cracking)
In genset, MRPP uses LPG to power up IC Engine and create electricity and Flue Gasses. Then the flue gasses will be used as a heater in the Pyrlosis. For the Pyrolysis, Azolla and Kohe (Kotoran Hewan) will be the input for Pyrolysis and then heated using flue gasses. The pyrolysis will be held in a reaction tank and then directed to a 4-series-condenser in which the result will be collected afterward. The results from the pyrolysis were biochar, biosyngas, and crude bio oil. In the MRPP itself, the products of pyrolysis (biochar, biosyngas, and bio oil) will then be processed forward with heating to around 400-550 Celcius degree. The end result will be the final product of biochar, biosyngas, and bio oil that can be used for usage.
There are still a lot improvements that can be made in MRPP especially regarding the FCC since the final product hasn't been finished for the project.
Overall, it was a pleasing experience since I saw the workshop and things in real life.
Here are some other pictures you can see from the workshop
FINAL EXAM AND PROJECT FOR HYDROGEN
| "Seeing and Feeling is BELIEVING" - DAI |
Midterm Progress can be seen in this section:
Abstract:
This study focused on the experimentation and analysis of hydrogen obtained through plate variation. The hydrogen was burned continuously using a one-way valve and small nozzle, resulting in a steady flame capable of boiling water. By measuring the temperature increase of water placed on a spoon, the energy gained from the experiment was determined. Additionally, the mechanical and electrical power generated were calculated. A comparison between the experimental and ideal conditions revealed slight discrepancies, likely due to the safety precautions of conducting the experiments in an open environment. These findings highlight the potential for energy production and emphasize the need for further optimization and refinement of experimental conditions to achieve more precise results.
For the full report, everything is written in here including the results and analyses: Regina Calysta Natalie's Electrolysis Report
On June 7, 2023, the study progressed by conducting experiments on the hydrogen obtained through plate variation, with a flow rate of 6.66666667e-7 kg/s. The research involved burning the hydrogen, which was safely distributed via a one-way valve and small nozzle. The continuous supply of hydrogen created a consistent flame capable of boiling water. The boiled water, placed on a spoon weighing 0.014 kg, experienced a temperature increase of approximately 27 degrees Celsius, rising from 28 degrees to 55 degrees. Upon calculations, it was determined that the experiment yielded a total of 1587.6 Joules of energy and 113,400 J/kg as a specific result.
The subsequent step involves inputting the actual values into the Open Modelica setting, enabling us to obtain the genuine results. Through modeling, it becomes apparent that the achievable mechanical power amounts to 0.0023 Watt, while the electric power reaches 0.0139 Watt. However, there is a slight loss when comparing these results to the simulation outcomes in Open Modelica. In an ideal scenario, the mechanical power could reach 0.0025 Watt, and the electrical power would be 0.015 Watt. Additionally, it is worth noting that the experiment conducted produced approximately 1600 Joules of energy, while the ideal conditions, as per the Matlab simulation, could generate approximately 9000 Joules or 0.0025 kWh. This loss can be attributed to conducting the experimentation in an open environment to ensure our own safety.
In conclusion, the experiment using an Aluminium 1100 cathode with a dimension of 5 x 10 mm and a total of 8 pieces, and varying the current between 6A, 8A, and 10A, with NaOH as the electrolyte, has proven the practicality and potential of electrolysis for generating hydrogen gas from water. There are 3 factors that play a part in the efficiency of how much hydrogen is produced: current flowing, material of the plates, dimension of the plates. More investigation and advancement in this area, including examining the impact of various electrode materials, optimising the current and voltage applied, and exploring the effects of different electrolyte concentrations, may lead to innovative and new uses of this technology, which can assist in addressing the challenges related to energy storage and sustainable energy production.
Furthermore, the experimentation process involved studying the hydrogen gained through plate variation, resulting in the continuous burning of hydrogen that produced a consistent flame capable of boiling water. The analysis of the boiled water revealed a significant increase in temperature. Further calculations showed the energy gained from the experiment, as well as the mechanical and electrical power produced. While there were slight discrepancies between the experimental and ideal conditions, these variations were expected due to the open environment in which the experiments were conducted for safety purposes. Overall, the study provided valuable insights into the potential energy production and demonstrated the importance of further refining and optimising the experimental conditions for more accurate results.
FINAL PROJECT SLIDES:
REGINA'S FINAL YEAR PROJECT SLIDES
VIDEO PRESENTATION:
FINAL PROJECT PRESENTATION