Difference between revisions of "Adam Muhammad Fadli"
(→05/04/2023) |
|||
| Line 90: | Line 90: | ||
[[File:ICEParameter.jpg|200px|thumb|center|Parameter]] | [[File:ICEParameter.jpg|200px|thumb|center|Parameter]] | ||
| − | The progress for my project | + | The progress for my project is shown in this slide: |
https://docs.google.com/presentation/d/1DUQ_vzmLys_-OkS9xjUD6ObYCHUsPLMlGuUdLUaA5NU/edit#slide=id.g22c7c873941_2_120 | https://docs.google.com/presentation/d/1DUQ_vzmLys_-OkS9xjUD6ObYCHUsPLMlGuUdLUaA5NU/edit#slide=id.g22c7c873941_2_120 | ||
| + | |||
| + | == Final Report == | ||
| + | For the creation of carbon-free hydrogen from nuclear and renewable sources, electrolysis is a potential alternative. The process of separating water into hydrogen and oxygen is known as electrolysis. This reaction occurs in a device known as an electrolyzer. Electrolyzers come in a variety of sizes, from little appliances that are ideal for small-scale dispersed hydrogen generation to massive central production plants that might be connected directly to non-greenhouse gas-generating sources of energy. | ||
| + | Electrolyzers are made up of an anode and a cathode that are separated by an electrolyte, similar to fuel cells. Different electrolyzers operate differently, mostly because of the various electrolyte materials used and the ionic species they conduct. The electrolyte of a polymer electrolyte membrane (PEM) electrolyzer is a solid specialized plastic substance. | ||
| + | From that, the water reacts at the anode to form oxygen and positively charged hydrogen ions (protons). After that the electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode. Lastly, at the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas. Anode Reaction: 2H2O → O2 + 4H+ + 4e- Cathode Reaction: 4H+ + 4e- → 2H2. | ||
| + | |||
| + | Through a thorough experimental method, this research seeks to explore the power efficiency of hydrogen electrolysis. We want to understand the characteristics that affect the efficiency of the electrolysis process by systematically adjusting important parameters such as current density, electrode materials, and electrolyte composition. In order to improve power efficiency and aid in the creation of more effective and sustainable hydrogen production systems, we also want to investigate the possibility of optimizing these characteristics. | ||
| + | |||
| + | In hydrogen electrolysis, water molecules are separated into hydrogen and oxygen gases using an electric current. An electrolyzer is used for the technique, which consists of two electrodes dipped in an electrolyte solution. While the cathode pulls positively charged hydrogen ions (H+), the anode draws negatively charged oxygen ions (O2-). The electric current in the electrolyzer promotes ion migration, which produces hydrogen gas at the cathode and oxygen gas at the anode. Hydrogen electrolysis's power efficiency is determined by the quantity of energy used to produce a certain amount of hydrogen gas. | ||
| + | Several variables affect the power efficiency of hydrogen electrolysis experiments. A crucial factor is the cell voltage, often known as the applied potential difference across the electrolyzer. Improved power efficiency with lower cell voltage is the consequence of fewer energy losses during the electrolysis process. Additionally, power efficiency is significantly impacted by the electrolyte utilized. Power efficiency rises as a result of more effective ion migration made possible by low-resistance conductive electrolytes. The design and components of the electrodes also affect overall efficiency. High-quality electrodes can accelerate hydrogen evolution while minimizing energy losses. They should have large surface areas and strong catalytic properties. | ||
| + | Although hydrogen electrolysis has great potential for long-term energy generation, there are still a number of obstacles that need to be cleared before its power efficiency can be increased any further. The high cost of catalyst materials is a significant barrier to widespread application. Research efforts should concentrate on creating affordable and effective catalysts to get over this obstacle. Another challenge is scaling up electrolysis systems without reducing effectiveness. This issue may be resolved with the use of technological advancements and economies of scale. Researching new electrode materials and electrolyte solutions with higher ion conductivity and catalytic activity may also help to boost power efficiency. | ||
| + | |||
| + | '''OpenModelica''' | ||
Latest revision as of 09:55, 13 June 2023
Contents
-Get To Know Me-
My name is Adam Muhammad Fadli with student ID 2006517745. You can call me Adam. I am 19 years old, I was born on 19 April 2003 in Jakarta, and am currently in my sixth semester at the University Of Indonesia.
My hobbies are playing basketball and games, I think the hobbies help my social skill.
Thank you for taking the time to read it.
-Chat session with CHATGPT-
Adam: Relation between sugar cane factory, automotive factory, and oil and gas factory? CHATGPT: There are several possible ways in which a sugar cane factory, an automotive factory, and an oil and gas factory could be related, depending on the specific context and circumstances. Here are a few examples:
1. Supply chain: One possible connection between these factories is that they could all be part of the same supply chain. For example, the sugar cane factory might supply sugar to the automotive factory, which uses it to make ethanol for fuel. The oil and gas factory might also supply fuel to the automotive factory, and could potentially use some of the ethanol as a blend component for gasoline. Additionally, the oil and gas factory might supply diesel or other fuels to power the sugar cane factory's machinery.
2. Energy consumption: Another possible connection is that all of these factories are likely to be large consumers of energy. The sugar cane factory needs the energy to power its machinery and to process the sugar cane into sugar or ethanol. The automotive factory needs fuel to power its assembly lines and other operations. The oil and gas factory needs the energy to extract, refine, and transport oil and gas. Depending on the location and energy sources available, these factories might also be connected in terms of their electricity or heat supply.
3. Environmental impacts: A third possible connection is that all of these factories have the potential to have significant environmental impacts. The sugar cane factory might generate waste products that need to be disposed of or might contribute to deforestation if it relies on clearing land for new plantations. The automotive factory might generate air or water pollution from its manufacturing processes, or contribute to greenhouse gas emissions through its use of fossil fuels. Of course, the oil and gas factory is directly involved in extracting and processing fossil fuels, which have a wide range of environmental impacts.
Overall, the specific relationship between these factories will depend on a variety of factors, such as their location, ownership structure, and the products they produce. However, it's clear that there are many potential connections and interdependencies between these different types of factories, and that they could have significant impacts on each other and on the wider economy and environment.
-Class Review- 24/02/2023
In yesterday's class, we learned to use OpenModelica to make a simulation for Internal Combustion Engine, We learned from Mr. Tanwir about the process of using OpenModelica. For the parameter, we use Thermosyspro as its guidance. But before we must download the library that is given in the OpenModelica library. The model is already pre-installed in the OpenModelica library.
CLASS SUMMARY 07/03/2023
Desalination is a process that removes mineral components from saline water(water containing a high concentration of dissolved salt). More generally, desalination refers to removing salts and minerals from a target substance. Saltwater (especially seawater) is desalinated to produce water suitable for humans to drink it. The way to make it ideal for humans is to filter it, one of the new innovations that have been discovered is using a graphene filter.
graphene is beneficial not just for desalination but also for utilizing carbon capture to address another environmental issue (CCU). The goal of CCU is to collect carbon dioxide (CO2) emissions from a variety of sources and transform them into beneficial goods like fuels, chemicals, or minerals. Biomass is a substance obtained from recently lived organisms and utilized in the production of bioenergy.
Because biomass is organic, it is composed of materials derived from living things like plants and animals. Plants, wood, and garbage are the most typical biomass resources utilized as energy sources. We refer to these as biomass feedstocks. A non-renewable energy source is also possible for biomass energy. an example of biomass is Biochar, what is actually biochar? In a regulated process known as pyrolysis, organic waste from forestry and agricultural operations is burned to produce biochar, a material that resembles charcoal. While it resembles regular charcoal in appearance, biochar is created using a particular method to reduce contamination and properly retain carbon.
-Introspection-
For the Class on 24 February, my friends and I are late to join Mr.Dai. I am really sorry for being late and will not repeat the same mistake again.
-Personal Project Progress-
-Comparing the efficiency of EV Car and EV Truck-
ECS 02 Personal Project Synopsis -https://youtu.be/61B-s7l3emk-
1. Synopsis The title will be called "Comparing the efficiency of EV Car and EV Truck". For the hands-on Project, I would like to do the topic of "electric energy to a mechanic energy". For the project, I will use data that is provided by their manufacturer. There will be a difference in efficiency due to the powertrain and weights. for the process to be done we need to determine:
It's worth noting that the efficiency of an electric vehicle can vary depending on several factors, such as driving conditions (e.g., speed, terrain), temperature, and battery age and health. Therefore, it's essential to conduct multiple tests under different conditions to get an accurate estimate of
The calculation of efficiency for EV cars and EV trucks can be different due to their different powertrain and weight. Here's a general process to calculate the difference in efficiency between an EV car and an EV truck:
1)Determine the energy consumption: To calculate the efficiency of an EV, you need to determine the amount of energy that the vehicle consumes to travel a certain distance. This can be done by measuring the battery capacity, the distance traveled, and the battery state of charge before and after the trip.
2)Calculate the energy efficiency: Once you have determined the energy consumption for both the EV car and truck, you can calculate their energy efficiency. Energy efficiency is calculated by dividing the distance traveled by the energy consumed. For example, if an EV car travels 100 miles using 30 kWh of energy, its energy efficiency is 100/30 = 3.33 miles per kWh.
3)Compare the efficiency: After calculating the energy efficiency for both the EV car and truck, you can compare their values. The vehicle with the higher energy efficiency is more efficient in using energy to travel a certain distance. This means it can travel farther on the same amount of energy compared to the other vehicle.
For the formula we can use: -Energy consumption : Energy used (kWh) / Distance traveled (miles or km) -Energy Efficiency: Distance traveled (miles or km) / Energy used (kWh) -Battery Capacity: Battery voltage (V) x Battery capacity (Ah) / 1000 -Range; Battery capacity (kWh) x Energy efficiency (miles/kWh or km/kWh)
Project Progress
Today me and some of my friends started doing the project by cutting the aluminum 1100 into plates, in the video, it can be seen the tools that we are using and the aluminum plate. For the video it can be seen from the link below: https://youtu.be/f5LR6YwSMK4
05/04/2023
Last Wednesday we went to Rasendriya's house to do the project, here is some understanding that I got during the project progress: Definition: Hydrogen electrolysis is a process that involves the use of electrical energy to split water molecules into their constituent atoms of hydrogen and oxygen. This process is carried out in an electrolysis cell that contains an electrolyte solution, typically a strong alkaline solution such as potassium hydroxide.
From the 3 experiments, the result that I got were 145, 140, and 110 from 3 different current and voltage
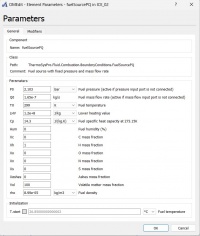
after that, we try it using OpenModelica the parameter that I got consisted of, input of the mass flow rate, fuel density, and the other parameter.
The progress for my project is shown in this slide: https://docs.google.com/presentation/d/1DUQ_vzmLys_-OkS9xjUD6ObYCHUsPLMlGuUdLUaA5NU/edit#slide=id.g22c7c873941_2_120
Final Report
For the creation of carbon-free hydrogen from nuclear and renewable sources, electrolysis is a potential alternative. The process of separating water into hydrogen and oxygen is known as electrolysis. This reaction occurs in a device known as an electrolyzer. Electrolyzers come in a variety of sizes, from little appliances that are ideal for small-scale dispersed hydrogen generation to massive central production plants that might be connected directly to non-greenhouse gas-generating sources of energy. Electrolyzers are made up of an anode and a cathode that are separated by an electrolyte, similar to fuel cells. Different electrolyzers operate differently, mostly because of the various electrolyte materials used and the ionic species they conduct. The electrolyte of a polymer electrolyte membrane (PEM) electrolyzer is a solid specialized plastic substance. From that, the water reacts at the anode to form oxygen and positively charged hydrogen ions (protons). After that the electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode. Lastly, at the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas. Anode Reaction: 2H2O → O2 + 4H+ + 4e- Cathode Reaction: 4H+ + 4e- → 2H2.
Through a thorough experimental method, this research seeks to explore the power efficiency of hydrogen electrolysis. We want to understand the characteristics that affect the efficiency of the electrolysis process by systematically adjusting important parameters such as current density, electrode materials, and electrolyte composition. In order to improve power efficiency and aid in the creation of more effective and sustainable hydrogen production systems, we also want to investigate the possibility of optimizing these characteristics.
In hydrogen electrolysis, water molecules are separated into hydrogen and oxygen gases using an electric current. An electrolyzer is used for the technique, which consists of two electrodes dipped in an electrolyte solution. While the cathode pulls positively charged hydrogen ions (H+), the anode draws negatively charged oxygen ions (O2-). The electric current in the electrolyzer promotes ion migration, which produces hydrogen gas at the cathode and oxygen gas at the anode. Hydrogen electrolysis's power efficiency is determined by the quantity of energy used to produce a certain amount of hydrogen gas. Several variables affect the power efficiency of hydrogen electrolysis experiments. A crucial factor is the cell voltage, often known as the applied potential difference across the electrolyzer. Improved power efficiency with lower cell voltage is the consequence of fewer energy losses during the electrolysis process. Additionally, power efficiency is significantly impacted by the electrolyte utilized. Power efficiency rises as a result of more effective ion migration made possible by low-resistance conductive electrolytes. The design and components of the electrodes also affect overall efficiency. High-quality electrodes can accelerate hydrogen evolution while minimizing energy losses. They should have large surface areas and strong catalytic properties. Although hydrogen electrolysis has great potential for long-term energy generation, there are still a number of obstacles that need to be cleared before its power efficiency can be increased any further. The high cost of catalyst materials is a significant barrier to widespread application. Research efforts should concentrate on creating affordable and effective catalysts to get over this obstacle. Another challenge is scaling up electrolysis systems without reducing effectiveness. This issue may be resolved with the use of technological advancements and economies of scale. Researching new electrode materials and electrolyte solutions with higher ion conductivity and catalytic activity may also help to boost power efficiency.
OpenModelica