Difference between revisions of "Muhammad Alif Kurniawan"
(→Functionality of hydrogen Tank) |
(→Material Selection) |
||
| (57 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
== '''Introduction''' == | == '''Introduction''' == | ||
| − | [[File:Muhammad Alif Kurniawan.jpg| | + | [[File:Muhammad Alif Kurniawan.jpg |500px|]] |
Hello, nice to meet all of you and welcome to my page | Hello, nice to meet all of you and welcome to my page | ||
| Line 64: | Line 64: | ||
All in all, hydrogen tanks play an important role in storing, transporting and using hydrogen as a source of clean energy. They enable broader and more efficient access to hydrogen, assist in reducing emissions and support the transition to a low-carbon economy. | All in all, hydrogen tanks play an important role in storing, transporting and using hydrogen as a source of clean energy. They enable broader and more efficient access to hydrogen, assist in reducing emissions and support the transition to a low-carbon economy. | ||
| − | == Advantages & Disadvantages of Hydrogen Tank == | + | == '''Advantages & Disadvantages of Hydrogen Tank''' == |
The use of hydrogen tanks as a fuel storage method certainly has some advantages and disadvantages. Here are some factors that can be considered in the hydrogen tank production process : | The use of hydrogen tanks as a fuel storage method certainly has some advantages and disadvantages. Here are some factors that can be considered in the hydrogen tank production process : | ||
| − | [[File:Fcto_storage_tree_chart2.png |500px|]] | + | [[File:Fcto_storage_tree_chart2.png |500px|]] [[File:Hydrogene_gris-bleu-vert_EN.jpg |700px|]] |
| Line 119: | Line 119: | ||
While hydrogen tanks have the advantage of being a sustainable energy storage, they also have some disadvantages that need to be addressed to drive wider adoption. Research and development is ongoing to address these challenges and improve the efficiency, safety and infrastructure associated with hydrogen tanks. | While hydrogen tanks have the advantage of being a sustainable energy storage, they also have some disadvantages that need to be addressed to drive wider adoption. Research and development is ongoing to address these challenges and improve the efficiency, safety and infrastructure associated with hydrogen tanks. | ||
| − | == Mass Calculation == | + | == '''Mass Calculation''' == |
| + | [[File:Hydrogen-project.jpg |430px|]] [[File:Gas projects Hydrogen (1).jpg |500px|]] | ||
| − | |||
| + | Hydrogen at a pressure of 8 bar has a boiling temperature of around -253.16° Celcius, therefore to be stable in liquid conditions a hydrogen tank with a lower temperature is needed. The total hydrogen mass in the tank cannot be determined using the ideal gas equation because the phase state possessed by hydrogen in the tank is liquid, while ideal gas will only function on the gas aspect. Therefore we will use the usual density equation which uses the density of liquid hydrogen in temperature -253.16° Celcius and and at a pressure of 0.8 MPa which is around 65.91 kg/m³. | ||
| − | ''' | + | |
| + | '''M = ρV''' | ||
Where: | Where: | ||
| − | + | M = Total Mass in kg | |
| + | |||
| + | ρ = Density in kg/m³ | ||
V = volume in m³ (cubic meter) | V = volume in m³ (cubic meter) | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | With the above equation, the total mass is obtained as follows : | |
| − | |||
| − | + | M = ρV | |
| + | M = (65.91)(0.001) | ||
| − | + | '''M = 0.06591 kg''' | |
| + | '''M = 65.91 gram''' | ||
| − | |||
| + | So, using this equation, the total mass of hydrogen in a tank with a volume of 1 liter, a pressure of 8 bar, and a temperature of -253.16°C is about '''0.06591 kilogram'''. | ||
| − | + | == '''Dimension Calculation''' == | |
| − | + | [[File:Hydrogen-1.jpg |585px|]] [[File:Hydrogen 2.png |585px|]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
To get the dimensions of a hydrogen tank that can accommodate hydrogen gas as much as possible and use as little material as possible, there are calculations that need to be done Using calculus and numerical methods, we will find out the dimensions of a hydrogen tank that has the maximum volume and minimum surface area by using the cylinder volume equation as follows: | To get the dimensions of a hydrogen tank that can accommodate hydrogen gas as much as possible and use as little material as possible, there are calculations that need to be done Using calculus and numerical methods, we will find out the dimensions of a hydrogen tank that has the maximum volume and minimum surface area by using the cylinder volume equation as follows: | ||
| Line 203: | Line 165: | ||
V = πr²h | V = πr²h | ||
| − | V/(πr²) = h | + | '''V/(πr²) = h''' |
| Line 215: | Line 177: | ||
A = 2πr² + 2πr(V/(πr²)) | A = 2πr² + 2πr(V/(πr²)) | ||
| − | A = 2πr² + 2V/r | + | '''A = 2πr² + 2V/r''' |
| Line 233: | Line 195: | ||
4πr = 2V/r² | 4πr = 2V/r² | ||
| − | 2πr³ = V | + | '''2πr³ = V''' |
| Line 245: | Line 207: | ||
d²(A)/dr² = 4π + 8π | d²(A)/dr² = 4π + 8π | ||
| − | d²(A)/dr² = 12π (PROVEN POSITIVE) | + | '''d²(A)/dr² = 12π (''PROVEN POSITIVE'')''' |
| Line 284: | Line 246: | ||
| − | Then the dimensions of the hydrogen tank to be used are '''(5.419 x 5.419 x 10.839) cm'''. This hydrogen tank will have a surface area of '''553,581 cm³''' | + | Then the dimensions of the hydrogen tank to be used are '''(5.419 x 5.419 x 10.839) cm'''. This hydrogen tank will have a surface area of '''553,581 cm²''' |
| + | |||
| + | == '''Material Selection''' == | ||
| + | |||
| + | When choosing a material for a hydrogen tank, there are several factors to consider. Some of the main factors to consider are: | ||
| + | |||
| + | |||
| + | 1. Corrosion Resistance | ||
| + | |||
| + | |||
| + | [[File:Corrosion-Resistance 1.jpg |500px|]] | ||
| + | |||
| + | |||
| + | Hydrogen has properties that can cause embrittlement or corrosion in some materials. Hydrogen corrosion can damage the material structure and reduce its strength. Therefore, in material selection, it is important to choose a material that has good resistance to hydrogen corrosion. The material often used for hydrogen tanks is special alloy steel such as nickel alloy steel or aluminum alloy steel. | ||
| + | |||
| + | |||
| + | 2. Mechanical Strength | ||
| + | |||
| + | |||
| + | [[File:Mechanical-Strength.jpg |500px|]] | ||
| + | |||
| + | |||
| + | Hydrogen tanks must be able to withstand the high pressures and associated mechanical loads. Therefore, the selected material must have adequate mechanical strength. Materials with high tensile strength and yield strength such as alloy steel or carbon fiber have been found to be suitable for hydrogen tank applications. | ||
| + | |||
| + | |||
| + | 3. Rigidity and Leakage | ||
| + | |||
| + | |||
| + | [[File:Rigidity-and-Leakage.jpg |500px|]] | ||
| + | |||
| + | |||
| + | Hydrogen tanks must have sufficient rigidity to prevent significant deformation when filled under high pressure. The material selected must have a high modulus of elasticity to maintain the structural rigidity of the tank. In addition, the material must have good ability to prevent leakage, either through pores or small cracks in the surface. The accuracy and smoothness of the material surface is also important to prevent hydrogen leakage. | ||
| + | |||
| + | |||
| + | 4. Compatibility | ||
| + | |||
| + | |||
| + | [[File:Compatibility.jpg |500px|]] | ||
| + | |||
| + | Material compatibility with hydrogen is also an important factor. Some materials can interact with hydrogen over long periods of time, causing harmful or harmful chemical reactions. It is important to choose a material that has good compatibility with hydrogen to prevent damage or leakage that can occur from a chemical reaction. | ||
| + | |||
| + | |||
| + | 5. Availability and Cost | ||
| + | |||
| + | |||
| + | [[File:Availability-and-Cost.jpg |500px|]] | ||
| + | |||
| + | |||
| + | Material availability and production costs also need to be considered in selecting hydrogen tank materials. Materials that are generally available and can be produced at an affordable cost would be a more practical choice. Alloy steels such as nickel alloy steel or aluminum alloy steel are generally easier to find and can be produced at a relatively affordable cost. | ||
| + | |||
| + | |||
| + | 6. Environmental and Sustainability Factors | ||
| + | |||
| + | |||
| + | [[File:Environmental_and_Sustainability_Factors.jpg |500px|]] | ||
| + | |||
| + | |||
| + | Apart from technical factors, it is also important to consider environmental and sustainability factors in material selection. Some materials may have a smaller environmental impact in their production and recycling. Choosing materials that are environmentally friendly and have a sustainable life cycle are important considerations in selecting hydrogen tank materials. | ||
| + | |||
| + | |||
| + | It is important to note that no single material perfectly satisfies all of the above factors. Therefore, material selection should be based on careful assessment and taking into account the specific requirements of a specific hydrogen tank application. Then by considering the several factors above with the specifications of the hydrogen tank owned, it was decided that the material to be used was '''Aluminum 7075''' and '''Aluminum 6061'''. | ||
| + | |||
| + | == '''The Most Suitable Material''' == | ||
| + | |||
| + | |||
| + | then to carry out the production process, several aspects need to be considered to choose the most suitable material between '''Aluminum 7075''' and '''Aluminum 6061''', this aspects can depend on a number of factors that need to be considered. Here are some factors to consider: | ||
| + | |||
| + | |||
| + | [[File:Aluminum-6061.jpg |500px|]] [[File:Aluminum-7075.jpg |500px|]] | ||
| + | |||
| + | |||
| + | 1. Strength and Durability | ||
| + | |||
| + | |||
| + | '''Aluminum 7075''' : Aluminum 7075 is an aluminum alloy with very high mechanical strength. It is a versatile alloy that is often used in the aerospace and military industries because of its high strength. Aluminum 7075 has a higher yield strength compared to Aluminum 6061, making it more suitable for applications requiring superior strength and durability. | ||
| + | |||
| + | '''Aluminum 6061''' : Aluminum 6061 is also a moderately strong aluminum alloy, but slightly lower in strength than Aluminum 7075. Aluminum 6061 is commonly used in a variety of applications, including the automotive and construction industries, due to its combination of good strength, sustainability and wide availability. | ||
| + | |||
| + | |||
| + | 2. Corrosion Resistance | ||
| + | |||
| + | |||
| + | '''Aluminum 7075''' : Aluminum 7075 has low corrosion properties, especially in harsh environments such as the marine or chemical industry. Although it has a lower corrosion resistance compared to some other aluminum alloys, Aluminum 7075 can be protected by coating its surface with a protective coating or special surface treatment. | ||
| + | |||
| + | '''Aluminum 6061''' : Aluminum 6061 has good corrosion resistance and is resistant to atmospheric and fresh water corrosion. This makes them suitable for a wide range of applications in generally less harsh environments. However, Aluminum 6061 may not be suitable for applications requiring high corrosion resistance. | ||
| + | |||
| + | |||
| + | 3. Surface Smoothness | ||
| + | |||
| + | |||
| + | '''Aluminum 7075''' : The surface of aluminum 7075 tends to have a higher roughness compared to Aluminum 6061. This surface roughness can affect the performance of the coating and the ability to avoid cracks in the material. Therefore, surface treatments such as sanding or special treatments may be required to achieve a smoother surface. | ||
| + | |||
| + | '''Aluminum 6061''' : The surface of aluminum 6061 is usually smoother than Aluminum 7075. This makes coating processes, such as anodizing or painting, easier and gives the final product a better appearance. | ||
| + | |||
| + | |||
| + | 4. Availability and Cost | ||
| + | |||
| + | |||
| + | '''Aluminum 7075''' : Aluminum 7075 is a more expensive aluminum alloy and can be difficult to find in some product variants. This is due to its strength properties and advantages in certain applications. This higher cost must be considered in the use of Aluminum 7075. | ||
| + | |||
| + | '''Aluminum 6061''' : Aluminum 6061 is more commonly available and more affordable than Aluminum 7075. Its wide availability and lower cost make it a more economical choice in most cases. | ||
| + | |||
| + | |||
| + | Of the 4 factors above, I decided to use '''Aluminum 6061''' material because of its superiority in Corrosion Resistance, Surface Smoothness, Availability and Cost compared to '''Aluminum 7075''' which only has advantages in Strength and Durability | ||
| + | |||
| + | == '''Production Cost''' == | ||
| + | |||
| + | [[File:Tankbayu.jpg |600px|]] [[File:Figure-2-visualizing-thickeness-and-composite-lay-up-build-up-in-acp-angle-2.jpg |600px|]] | ||
| + | |||
| + | |||
| + | To consider production costs, we need to consider material costs first, according to the standards set by the American Society of Mechanical Engineers (ASME), a hydrogen tank has a thickness of about 4-6 mm, to take a safe point we need to take the maximum thickness, then the material used can be considered with the following calculation : | ||
| + | |||
| + | |||
| + | '''Thickness Volume = A . dr''' | ||
| + | |||
| + | |||
| + | The surface area equation that we will use is the minimum surface area equation obtained when determining the dimensions, which are as follows : | ||
| + | |||
| + | |||
| + | A = 2πr² + 2πrh | ||
| + | |||
| + | A = 2πr² + 2πr(2r) | ||
| + | |||
| + | '''A = 6πr²''' | ||
| + | |||
| + | |||
| + | Then the integral process for the value of r in units of cm up to '''r' = r + 0.6''' in units of cm, can be described as follows | ||
| + | |||
| + | |||
| + | '''Thickness Volume = A [r, r'] . dr''' | ||
| + | |||
| + | V = 6πr² [r, r+0.6] . dr | ||
| + | |||
| + | V = ∫[r, r+0.6] 6πr² dr | ||
| + | |||
| + | V = 6π . ∫[r, r+0.6] r² dr | ||
| + | |||
| + | V = 6π . (1/3) . [r, r+0.6]³ | ||
| + | |||
| + | V = 2π . ((r+0.6)³ - r³) | ||
| + | |||
| + | V = 2π . ((5.419+0.6)³ - 5.419³) | ||
| + | |||
| + | '''V = 370.28 cm³''' | ||
| + | |||
| + | '''V = 0.00037028 m³''' | ||
| + | |||
| + | |||
| + | Then by using Aluminum 6061 density of '''2720 kg/m³''', the mass of material needed for the production of one hydrogen tank is obtained as follows : | ||
| + | |||
| + | |||
| + | '''M = ρV''' | ||
| + | |||
| + | |||
| + | Where: | ||
| + | |||
| + | M = Total Mass in kg | ||
| + | |||
| + | ρ = Density in kg/m³ | ||
| + | |||
| + | V = volume in m³ (cubic meter) | ||
| + | |||
| + | |||
| + | With the above equation, the total mass is obtained as follows : | ||
| + | |||
| + | |||
| + | M = ρV | ||
| + | |||
| + | M = (2720)(0.00037028) | ||
| + | |||
| + | '''M = 1.007 kg''' | ||
| + | |||
| + | |||
| + | So, using this equation, the total mass of Aluminum 6061 in every hydrogen tank is around '''1.007 kilogram'''. After that, if we consider the cost of Aluminum 6061 material is '''''USD 2.4 / kg''''' or '''''IDR 35,779 / kg'''''. Then the material costs per hydrogen tank will be obtained as follows : | ||
| + | |||
| + | |||
| + | '''Material Cost = M . P''' | ||
| + | |||
| + | MC = (1.007)(35.779) | ||
| + | |||
| + | '''MC = IDR 36,035 / Hydrogen Tank''' | ||
| + | |||
| + | |||
| + | The percentage of production costs used for materials on average from total expenditure may vary depending on the industry and type of production being discussed. However, in general, this percentage can range from '''30%''' to '''70%''' of total expenses. Using this range, the total expenditure range for the production of hydrogen tanks is around '''''IDR 51,479 - 120,117 per hydrogen tank'''''. | ||
| + | |||
| + | |||
| + | So on average, the production cost will only take '''''IDR 85.798 per hydrogen tank''''' This price range is still within the range of client requests, which is below '''''IDR 500,000''''', it means that the design cost for this production is acceptable and the production process will be approved so that the manufacturing process will be able to run without a hitch. | ||
Latest revision as of 13:54, 12 June 2023
Contents
Introduction
Hello, nice to meet all of you and welcome to my page
My name is Muhammad Alif Kurniawan with NPM 2106732853
You can ask anything, lets going forward together
Definition of Hydrogen Tank
First of all, we will discuss a simple definition of a hydrogen tank, we can make a brief explanation about a hydrogen tank as follows :
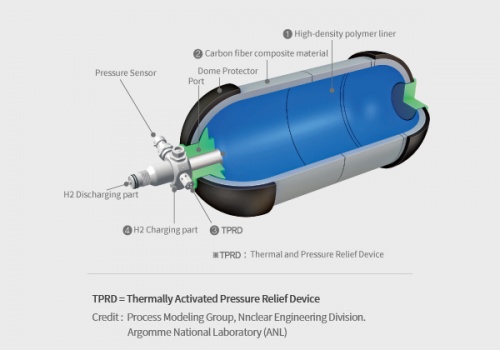
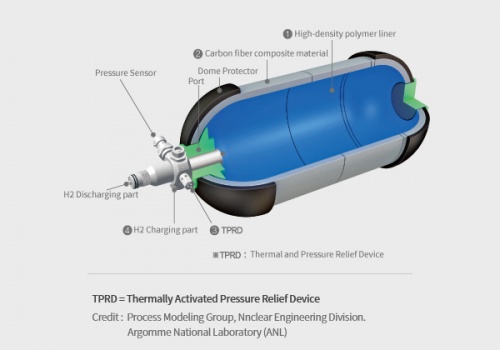
A hydrogen tank is a specialized container designed to store hydrogen gas, either under high pressure or as a cryogenic liquid, for various applications such as fuel cell vehicles, power generation, and industrial processes. The tank's primary function is to safely store hydrogen until it is needed and then release it for use in the desired application. It is constructed using high-strength materials to withstand the pressure or low-temperature conditions required for hydrogen storage. The tank may incorporate safety features, insulation (in the case of cryogenic tanks), and fittings or valves for filling and dispensing hydrogen.
After that, based on the assignment given on May 22, 2023. Every students are assigned to make an optimal design design for a hydrogen tank, for for the specifications that are used as constant variables in the production of hydrogen tanks can be seen below :
1. Has a capacity of 1 Liter
2. Operates at a pressure of 8 bar
3. Have a maximum production price of Rp. 500,000
Functionality of hydrogen Tank
Hydrogen tanks have been used in various ways, be it in everyday life or for other purposes, be it small scale to industrial scale. Here are the three main functionalities of a hydrogen tank:
A. Storage and Transport
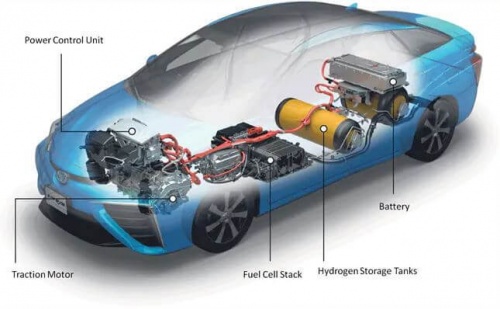
The main function of a hydrogen tank is to store and transport hydrogen gas safely. These tanks allow for the storage of significant amounts of hydrogen for use in applications such as fuel cell vehicles, power generation and industrial processes. By storing a sufficient amount of hydrogen, tanks allow efficient transportation from one location to another, allowing access to renewable energy sources in certain places.
B. Clean Energy Source
Hydrogen is a clean energy source and can be used to reduce greenhouse gas emissions and air pollution. By using a hydrogen tank, energy stored in the form of hydrogen can be released and used to generate electricity via fuel cells, drive fuel cell vehicles, or meet industrial energy needs. Hydrogen tanks play an important role in providing access to an environmentally friendly and sustainable source of energy.
C. Integration with Energy Infrastructure
Hydrogen tanks also work within the integration of a wider energy infrastructure. They can be connected to a hydrogen dispensing system which enables efficient refueling of fuel cell vehicles. In addition, hydrogen tanks can be integrated with existing energy storage networks and natural gas networks to provide energy reserves, offset fluctuations in supply and demand, and strengthen the stability of the energy system.
All in all, hydrogen tanks play an important role in storing, transporting and using hydrogen as a source of clean energy. They enable broader and more efficient access to hydrogen, assist in reducing emissions and support the transition to a low-carbon economy.
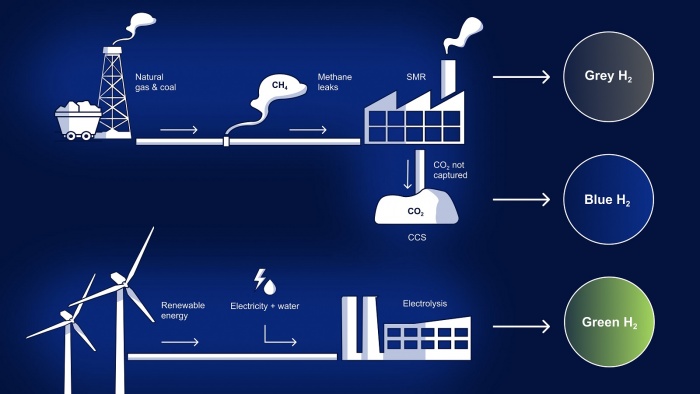
Advantages & Disadvantages of Hydrogen Tank
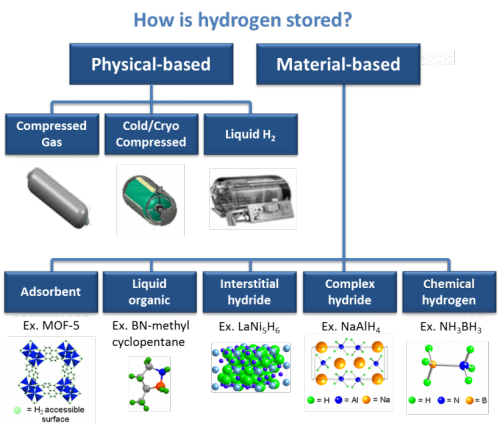
The use of hydrogen tanks as a fuel storage method certainly has some advantages and disadvantages. Here are some factors that can be considered in the hydrogen tank production process :
ADVANTAGES
1. Energy Sustainability
Hydrogen tanks allow for sustainable energy storage. Hydrogen can be produced from renewable energy sources, such as solar or wind power, through the electrolysis of water. By storing the generated energy in the form of hydrogen, the tank allows long-term storage and use of renewable energy when needed. This helps overcome the challenges of fluctuating energy supply and demand and enables the wider use of renewable energy.
2. High Energy Capacity
Hydrogen tanks have a very high energy capacity compared to conventional batteries. Relatively lighter in weight, a hydrogen tank can store more energy than a battery of the same capacity. This allows fuel cell vehicles to have a wider range and faster refueling times. In addition, the high energy capacity of hydrogen tanks also allows energy storage and use on a larger industrial scale.
3. Fast Charging Time
Filling a hydrogen tank can be done in a relatively short time. Compared to charging an electric battery which requires a longer time, filling a hydrogen tank can be comparable to filling a conventional fuel. This allows the use of fuel cell vehicles that are more comfortable and similar to fossil fuel vehicles. This fast charging time also contributes to efficiency and convenience in the use of hydrogen energy.
With energy sustainability, high energy capacity and fast filling times, hydrogen tanks are an attractive solution for energy storage and the use of hydrogen as a clean fuel. These advantages are helping to drive the adoption of hydrogen as a promising alternative in the transition to a more sustainable energy system.
DISADVANTAGES
1. High Pressure or Temperature
Hydrogen tanks used to store hydrogen in gaseous form usually require high pressure, reaching thousands of psi (pounds per square inch) to store sizable quantities. This requires a robust and high pressure resistant tank design, and creates a safety risk that must be managed with care. On the other hand, hydrogen tanks that store hydrogen as a cryogenic liquid require extremely low temperatures, below -253 degrees Celsius (-423 degrees Fahrenheit), which require additional insulation and infrastructure to maintain those low temperatures. Both of these can pose significant technical and cost challenges.
2. Storage Disadvantages
Although the energy capacity of hydrogen in a tank can be high, the energy stored in hydrogen has a disadvantage in terms of volume. Hydrogen has a low energy density when compared to fossil fuels such as gasoline or diesel. This means the hydrogen tank must be larger in size or heavier in order to store the same amount of energy compared to conventional fuels. Storage space-consuming can be a challenge in some applications, such as vehicles, where limited space is an important consideration.
3. Limited Infrastructure
Infrastructure for the production, storage and distribution of hydrogen is still limited and not fully developed as widely as fossil fuel infrastructure. This means limited access to hydrogen filling stations, lack of a distribution network, and lack of hydrogen production facilities can all become barriers to wider adoption of hydrogen-based technologies. Developing the infrastructure needed to support hydrogen tanks requires a large investment and takes a long time.
While hydrogen tanks have the advantage of being a sustainable energy storage, they also have some disadvantages that need to be addressed to drive wider adoption. Research and development is ongoing to address these challenges and improve the efficiency, safety and infrastructure associated with hydrogen tanks.
Mass Calculation
Hydrogen at a pressure of 8 bar has a boiling temperature of around -253.16° Celcius, therefore to be stable in liquid conditions a hydrogen tank with a lower temperature is needed. The total hydrogen mass in the tank cannot be determined using the ideal gas equation because the phase state possessed by hydrogen in the tank is liquid, while ideal gas will only function on the gas aspect. Therefore we will use the usual density equation which uses the density of liquid hydrogen in temperature -253.16° Celcius and and at a pressure of 0.8 MPa which is around 65.91 kg/m³.
M = ρV
Where:
M = Total Mass in kg
ρ = Density in kg/m³
V = volume in m³ (cubic meter)
With the above equation, the total mass is obtained as follows :
M = ρV
M = (65.91)(0.001)
M = 0.06591 kg
M = 65.91 gram
So, using this equation, the total mass of hydrogen in a tank with a volume of 1 liter, a pressure of 8 bar, and a temperature of -253.16°C is about 0.06591 kilogram.
Dimension Calculation
To get the dimensions of a hydrogen tank that can accommodate hydrogen gas as much as possible and use as little material as possible, there are calculations that need to be done Using calculus and numerical methods, we will find out the dimensions of a hydrogen tank that has the maximum volume and minimum surface area by using the cylinder volume equation as follows:
Volume = πr²h
V = πr²h
V/(πr²) = h
Then combining it with the equation for the surface area of the tube will get a new equation form, namely :
Surface Area = 2πr² + 2πrh
A = 2πr² + 2πrh
A = 2πr² + 2πr(V/(πr²))
A = 2πr² + 2V/r
Now, we need to find the minimum value of the surface area formula. We can use calculus to find the first and second derivatives of the formula.
d(A)/dr = 4πr - 2V/r²
d²(A)/dr² = 4π + 4V/r³
To find the minimum, we look for the point where the first derivative equals zero and the second derivative is positive (to confirm that it is a minimum value).
4πr - 2V/r² = 0
4πr = 2V/r²
2πr³ = V
To be sure, if we plug in the values we already got in the second derivative equation we will get the following values
d²(A)/dr² = 4π + 4V/r³
d²(A)/dr² = 4π + 4(2πr³)/r³
d²(A)/dr² = 4π + 8π
d²(A)/dr² = 12π (PROVEN POSITIVE)
To determine the relationship between the radius (r) and the height (h), we can use the initial volume equation
h = V/(πr²)
h = 2πr³/(πr²)
h = 2r
Using this equation, we will find the effective dimensions for a hydrogen tank with a volume capacity of 1 liter (1000 cm³) as follows :
V = πr²h
V = πr²(2r)
V = 2πr³
r³ = (V/2π)
r = (1000/2π)^(1/3)
r = 5.419 cm
Then for the height of the tube obtained
h = 2r
h = (2)(5.419)
h = 10.839 cm
Then the dimensions of the hydrogen tank to be used are (5.419 x 5.419 x 10.839) cm. This hydrogen tank will have a surface area of 553,581 cm²
Material Selection
When choosing a material for a hydrogen tank, there are several factors to consider. Some of the main factors to consider are:
1. Corrosion Resistance
Hydrogen has properties that can cause embrittlement or corrosion in some materials. Hydrogen corrosion can damage the material structure and reduce its strength. Therefore, in material selection, it is important to choose a material that has good resistance to hydrogen corrosion. The material often used for hydrogen tanks is special alloy steel such as nickel alloy steel or aluminum alloy steel.
2. Mechanical Strength
Hydrogen tanks must be able to withstand the high pressures and associated mechanical loads. Therefore, the selected material must have adequate mechanical strength. Materials with high tensile strength and yield strength such as alloy steel or carbon fiber have been found to be suitable for hydrogen tank applications.
3. Rigidity and Leakage
Hydrogen tanks must have sufficient rigidity to prevent significant deformation when filled under high pressure. The material selected must have a high modulus of elasticity to maintain the structural rigidity of the tank. In addition, the material must have good ability to prevent leakage, either through pores or small cracks in the surface. The accuracy and smoothness of the material surface is also important to prevent hydrogen leakage.
4. Compatibility
Material compatibility with hydrogen is also an important factor. Some materials can interact with hydrogen over long periods of time, causing harmful or harmful chemical reactions. It is important to choose a material that has good compatibility with hydrogen to prevent damage or leakage that can occur from a chemical reaction.
5. Availability and Cost
Material availability and production costs also need to be considered in selecting hydrogen tank materials. Materials that are generally available and can be produced at an affordable cost would be a more practical choice. Alloy steels such as nickel alloy steel or aluminum alloy steel are generally easier to find and can be produced at a relatively affordable cost.
6. Environmental and Sustainability Factors
Apart from technical factors, it is also important to consider environmental and sustainability factors in material selection. Some materials may have a smaller environmental impact in their production and recycling. Choosing materials that are environmentally friendly and have a sustainable life cycle are important considerations in selecting hydrogen tank materials.
It is important to note that no single material perfectly satisfies all of the above factors. Therefore, material selection should be based on careful assessment and taking into account the specific requirements of a specific hydrogen tank application. Then by considering the several factors above with the specifications of the hydrogen tank owned, it was decided that the material to be used was Aluminum 7075 and Aluminum 6061.
The Most Suitable Material
then to carry out the production process, several aspects need to be considered to choose the most suitable material between Aluminum 7075 and Aluminum 6061, this aspects can depend on a number of factors that need to be considered. Here are some factors to consider:
1. Strength and Durability
Aluminum 7075 : Aluminum 7075 is an aluminum alloy with very high mechanical strength. It is a versatile alloy that is often used in the aerospace and military industries because of its high strength. Aluminum 7075 has a higher yield strength compared to Aluminum 6061, making it more suitable for applications requiring superior strength and durability.
Aluminum 6061 : Aluminum 6061 is also a moderately strong aluminum alloy, but slightly lower in strength than Aluminum 7075. Aluminum 6061 is commonly used in a variety of applications, including the automotive and construction industries, due to its combination of good strength, sustainability and wide availability.
2. Corrosion Resistance
Aluminum 7075 : Aluminum 7075 has low corrosion properties, especially in harsh environments such as the marine or chemical industry. Although it has a lower corrosion resistance compared to some other aluminum alloys, Aluminum 7075 can be protected by coating its surface with a protective coating or special surface treatment.
Aluminum 6061 : Aluminum 6061 has good corrosion resistance and is resistant to atmospheric and fresh water corrosion. This makes them suitable for a wide range of applications in generally less harsh environments. However, Aluminum 6061 may not be suitable for applications requiring high corrosion resistance.
3. Surface Smoothness
Aluminum 7075 : The surface of aluminum 7075 tends to have a higher roughness compared to Aluminum 6061. This surface roughness can affect the performance of the coating and the ability to avoid cracks in the material. Therefore, surface treatments such as sanding or special treatments may be required to achieve a smoother surface.
Aluminum 6061 : The surface of aluminum 6061 is usually smoother than Aluminum 7075. This makes coating processes, such as anodizing or painting, easier and gives the final product a better appearance.
4. Availability and Cost
Aluminum 7075 : Aluminum 7075 is a more expensive aluminum alloy and can be difficult to find in some product variants. This is due to its strength properties and advantages in certain applications. This higher cost must be considered in the use of Aluminum 7075.
Aluminum 6061 : Aluminum 6061 is more commonly available and more affordable than Aluminum 7075. Its wide availability and lower cost make it a more economical choice in most cases.
Of the 4 factors above, I decided to use Aluminum 6061 material because of its superiority in Corrosion Resistance, Surface Smoothness, Availability and Cost compared to Aluminum 7075 which only has advantages in Strength and Durability
Production Cost
To consider production costs, we need to consider material costs first, according to the standards set by the American Society of Mechanical Engineers (ASME), a hydrogen tank has a thickness of about 4-6 mm, to take a safe point we need to take the maximum thickness, then the material used can be considered with the following calculation :
Thickness Volume = A . dr
The surface area equation that we will use is the minimum surface area equation obtained when determining the dimensions, which are as follows :
A = 2πr² + 2πrh
A = 2πr² + 2πr(2r)
A = 6πr²
Then the integral process for the value of r in units of cm up to r' = r + 0.6 in units of cm, can be described as follows
Thickness Volume = A [r, r'] . dr
V = 6πr² [r, r+0.6] . dr
V = ∫[r, r+0.6] 6πr² dr
V = 6π . ∫[r, r+0.6] r² dr
V = 6π . (1/3) . [r, r+0.6]³
V = 2π . ((r+0.6)³ - r³)
V = 2π . ((5.419+0.6)³ - 5.419³)
V = 370.28 cm³
V = 0.00037028 m³
Then by using Aluminum 6061 density of 2720 kg/m³, the mass of material needed for the production of one hydrogen tank is obtained as follows :
M = ρV
Where:
M = Total Mass in kg
ρ = Density in kg/m³
V = volume in m³ (cubic meter)
With the above equation, the total mass is obtained as follows :
M = ρV
M = (2720)(0.00037028)
M = 1.007 kg
So, using this equation, the total mass of Aluminum 6061 in every hydrogen tank is around 1.007 kilogram. After that, if we consider the cost of Aluminum 6061 material is USD 2.4 / kg or IDR 35,779 / kg. Then the material costs per hydrogen tank will be obtained as follows :
Material Cost = M . P
MC = (1.007)(35.779)
MC = IDR 36,035 / Hydrogen Tank
The percentage of production costs used for materials on average from total expenditure may vary depending on the industry and type of production being discussed. However, in general, this percentage can range from 30% to 70% of total expenses. Using this range, the total expenditure range for the production of hydrogen tanks is around IDR 51,479 - 120,117 per hydrogen tank.
So on average, the production cost will only take IDR 85.798 per hydrogen tank This price range is still within the range of client requests, which is below IDR 500,000, it means that the design cost for this production is acceptable and the production process will be approved so that the manufacturing process will be able to run without a hitch.