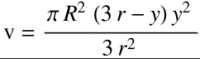Difference between revisions of "Ridwan Sholehan"
(→Study Case: Optimization Hydrogen Storage) |
(→Study Case: Optimization Hydrogen Storage) |
||
| Line 48: | Line 48: | ||
4. nanostructured materials (adsorption of molecular hydrogen) | 4. nanostructured materials (adsorption of molecular hydrogen) | ||
| − | |||
Refers to the type above. There is a material that can be used to build storage. Hydrogen Magnesium Hydride (MgH2) storage material by inserting a double catalyst, namely Iron Oxide (Fe2O3) and Silicon Carbide (SiC) each of 5wt% as an effort to improve the absorption properties and reaction kinetics of magnesium-based hydrogen storage materials. | Refers to the type above. There is a material that can be used to build storage. Hydrogen Magnesium Hydride (MgH2) storage material by inserting a double catalyst, namely Iron Oxide (Fe2O3) and Silicon Carbide (SiC) each of 5wt% as an effort to improve the absorption properties and reaction kinetics of magnesium-based hydrogen storage materials. | ||
| Line 56: | Line 55: | ||
[[File:Storage Tank.png|200x200px]] | [[File:Storage Tank.png|200x200px]] | ||
| − | + | This is a reference to the design of the storage hydrogen Tank. this design has several advantages because it will be installed in a vehicle it has to be lightweight, high durability, and efficient to be used in the vehicle. | |
'''Calculation of Hydrogen Storage''' | '''Calculation of Hydrogen Storage''' | ||
| Line 71: | Line 70: | ||
r : 5 cm = 0.05 m | r : 5 cm = 0.05 m | ||
| − | + | For the bottom and top of a vertical tank, for provided values for R and r as described above, and content bisector y, with numerical ranges of 0 <= y <= r, and L+r <= y <= L+2r (with some technical adjustments of the range of y and of the resulting volume for the top ellipse): | |
| − | |||
| − | + | [[File:Screenshot 2023-05-29 092048.png|200x200px]] | |
| − | + | And for the cylinder in the middle, for a provided value of R (cylinder radius) and with the numerical range of r <= y <= L+r | |
| − | + | [[File:Screenshot_2023-05-29_092547.png|200x200px]] | |
| − | + | So we can get L from the equation above: | |
| − | + | y = 0.081 m | |
'''Tank Testing and Safety Consideration''' | '''Tank Testing and Safety Consideration''' | ||
Revision as of 20:42, 4 June 2023
INTRODUCTION
Nama :Ridwan Sholehan
NPM :2206100312
Motto Hidup:
"Jangan Terlalu Terpaku Akan Suatu Penyesalan, Yang Terpenting Adalah Bagaimana Langkah Untuk Membangun Masa Depan Yang Lebih Baik"
Study Case: Optimization Hydrogen Storage
Hydrogen Storage Requirement
In this case we want to build a storage with:
1. Capacity : 1 Liter.
2. Pressure : 8 Bar.
3. Budget : Rp. 500.000,-
4. Can be installed in a vehicle such as a motorcycle.
Material Classification
Hydrogen storage materials can be of different types:
1. dissociative material in which molecular hydrogen is dissociated into hydrogen atoms, which occupy interstitial sites.
2. material with chemically bound hydrogen.
3. materials that adsorb molecular hydrogen.
Hydrogen storage in solid form can be briefly classified into the following categories:
1. metal hydrides
2. light metal-based hydrides
3. chemical hydrides (complex hydrides)
4. nanostructured materials (adsorption of molecular hydrogen)
Refers to the type above. There is a material that can be used to build storage. Hydrogen Magnesium Hydride (MgH2) storage material by inserting a double catalyst, namely Iron Oxide (Fe2O3) and Silicon Carbide (SiC) each of 5wt% as an effort to improve the absorption properties and reaction kinetics of magnesium-based hydrogen storage materials.
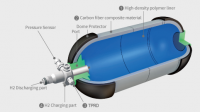
Design of Storage Tank
This is a reference to the design of the storage hydrogen Tank. this design has several advantages because it will be installed in a vehicle it has to be lightweight, high durability, and efficient to be used in the vehicle.
Calculation of Hydrogen Storage
Specification:
1. Capacity --> Volume : 1 liter = 0.001 m^3
2. Because this storage will be installed in vehicles, which means having limited space. we can assume for :
R : 10 cm = 0.1 m
r : 5 cm = 0.05 m
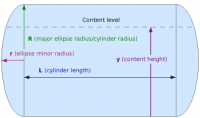
For the bottom and top of a vertical tank, for provided values for R and r as described above, and content bisector y, with numerical ranges of 0 <= y <= r, and L+r <= y <= L+2r (with some technical adjustments of the range of y and of the resulting volume for the top ellipse):
And for the cylinder in the middle, for a provided value of R (cylinder radius) and with the numerical range of r <= y <= L+r
So we can get L from the equation above:
y = 0.081 m
Tank Testing and Safety Consideration
In accordance with ISO/TS 15869 (revised):
1. Burst test: the pressure at which the tank bursts, typically more than 2× the working pressure.
2. Proof pressure: the pressure at which the test will be executed, typically above the working pressure.
3. Leak test or permeation test,[9] in NmL/hr/L (Normal liter of H2/time in hr/volume of the tank.)
4. Fatigue test, typically several thousand cycles of charging/emptying.
5. Bonfire test where the tank is exposed to an open fire.
6. Bullet test where live ammunition is fired at the tank.